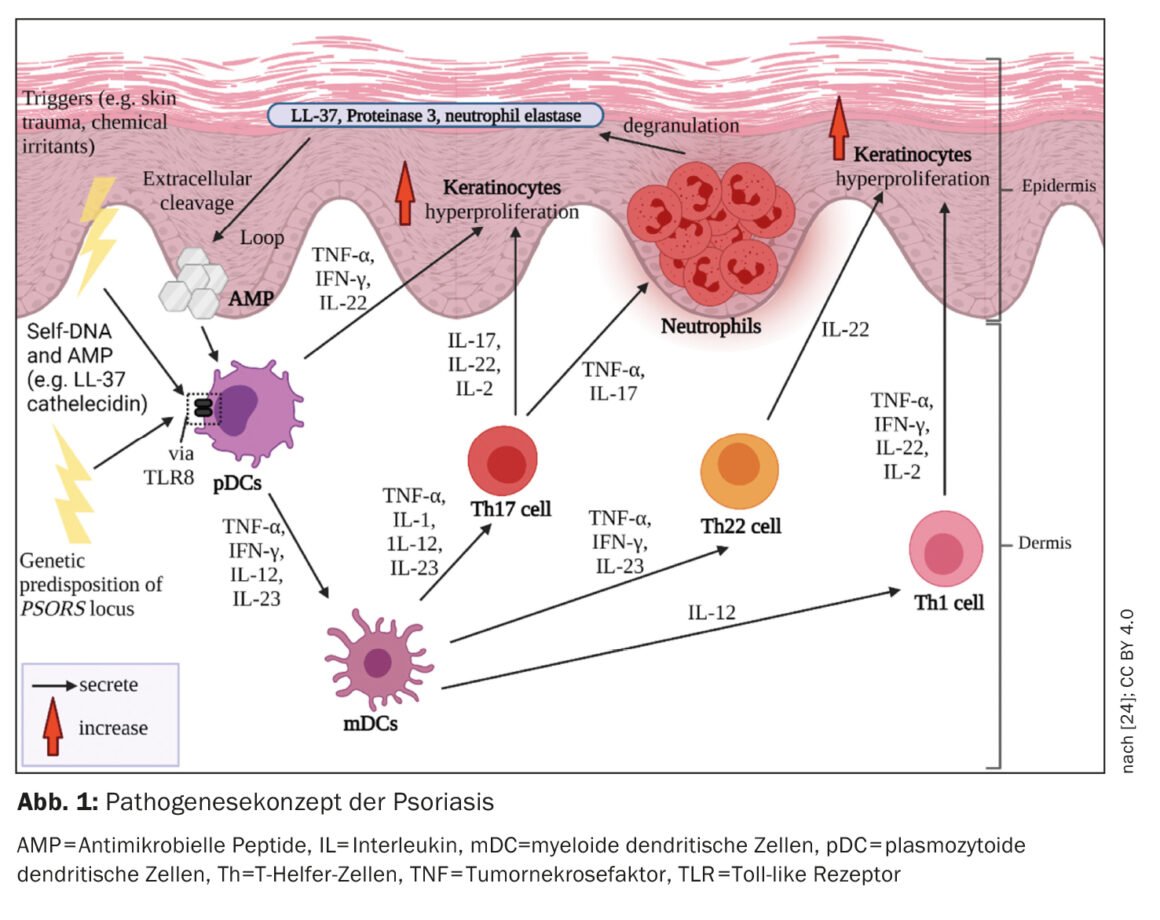
The innovative systemic treatments for psoriasis are a prime example of translational medicine. In a review published last year in the Journal of Investigative Dermatology, Prof. Peter van de Kerkhof, MD, former president of the European Society for Dermatological Research (ESDR) and chief medical officer at the International Psoriasis Council, provides an update on therapy-relevant immunopathogenetic concepts in psoriasis.
In recent decades, considerable progress has been made in elucidating the complex and multifactorial pathogenesis of psoriasis as a basis for the development of highly effective therapeutic options [1]. Today, at the cellular and molecular level, a T cell-mediated immune response and an associated characteristic cytokine milieu are considered to be the essential pathomechanism [2]. At the genetic level, numerous gene loci associated with psoriasis have been identified. HLA-C*06:02 is considered to be the main risk allele of psoriasis vulgaris [3]. This is located on the psoriasis susceptibility locus PSORS1. Evidence for autoimmune pathomechanisms in psoriasis was provided by the finding that HLA-C*06:02 elicited an autoimmune response against ADAMTSL5, a melanocytic autoantigen [17,18]. In addition to ADAMTSL5, LL-37/cathelicidin and PLA2G4D are two other potential autoantigens that appear to play a role in the initiation or maintenance of psoriatic lesions [19].
Psoriasis as a T-cell mediated autoimmune disease
Processes of the innate and acquired immune system are involved in the immunopathogenesis of psoriasis (Fig. 1). Involved in the onset of the inflammatory cascade are cells of the innate immune system. In this regard, dendritic cells (DC) and macrophages appear to play a key role in the establishment of an immune response dominated by T helper cells-1 (Th1) and Th17. Activation of DC in the skin leads to the release of interleukins-12 (IL-12) and IL-23, which stimulate naïve T cells to differentiate into Th1 (by IL-12) and Th17 (by IL-23) cells [4]. T helper (Th) cells coordinate the cell-mediated adaptive immune response. Activated Th1 and Th17 cells secrete the cytokines IFN-γ and TNF-α and IL-17 and IL-22, respectively, which drive further pathogenesis [5,6].
Proinflammatory environment
In psoriasis, the immune response is mediated mainly by Th1 cells. whereas in atopic dermatitis there is Th2 dominance. T-cell populations in biopsies of psoriatic lesions, which showed different T-cell infiltrates compared with lesions in the setting of atopic dermatitis in the same patient, confirm the Th1/Th2 paradigm [1,8]. TNF-α, IFN-γ, IL-17A, IL-17F, IL-22, and IL-6 form a cytokine network in psoriatic lesions that induces hyperproliferation of keratinocytes, among others [1]. The accelerated differentiation process of keratinocytes shortens their transit time from the stratum basale to the stratum corneum from about 30 to 5-8 days [9]. This results in the scaling and thickening of the epidermis typical of psoriasis. Activated keratinocytes also produce proinflammatory mediators and express adhesion molecules on the cell surface, leading to amplification and chronification of the immune response [10].
Immunological memory: “Tissue Resident Memory T cells”.
Psoriatic lesions have a higher total immune cell count compared to healthy skin [11]. Even in the skin of psoriasis patients, which clinically shows no lesions, a higher number of T cells could be detected than in healthy skin [11]. Intraepidermal accumulation of CD8 cells is crucial for the development of psoriatic lesions [1]. But how does it come about? After autoantigens are presented to dendritic cells, T cell activation occurs. Activated T cells migrate into the epidermis, requiring complex processes to enter the epidermis – α1β1-integrins are thought to play an important role in this process. Blockade of α1β1 by a monoclonal antibody resulted in the accumulation of epidermal T cells and the development of psoriatic lesions in mouse models [12].
T cells located in the epidermis play an extremely important role in the pathogenesis of psoriasis. In psoriatic plaques, CD4-positive cells are found primarily in the dermis and CD8-positive cells are found predominantly in the epidermis [13]. A large proportion of CD8-positive cells in the epidermis belong to the “Tissue Resident Memory T cells” (TRM cells, CD8+ TRM). These are memory T cells that remain in cleared psoriatic lesions and maintain an immunological memory, which explains why psoriatic lesions often recur at the same sites [14]. CD8+ TRM are a potential biomarker of residual disease activity [15,16].
Medical history review: antipsoriatic systemic therapies.
In the 1980s, studies with the immunosuppressant ciclosporin A, which proved effective in the treatment of psoriasis, confirmed that it was a T-cell mediated disease [26,27]. Ciclosporin inhibits the phosphatase calcineurin in the cytoplasm, thereby blocking the phosphorylation of nuclear factor for T cells (NFATc), which is responsible for activating the genes for various proinflammatory cytokines [1]. Further support for the proposition that T cells are an important therapeutic target was provided by the antipsoriatic effects of anti-CD4 and CTLA-4-Ig [28,29]. In the 1990s, a US research group showed that autologous T cell injection could induce lesions in non-diseased psoriatic skin grafted onto SCID mice [30]. In the same model, CD4+ T lymphocytes were shown to induce psoriatic lesions in non-diseased psoriatic skin [31].
| Monoclonal antibodies and small molecules Biologics have revolutionized the treatment options for psoriasis. With the biological active substances available today, long-term effective and safe disease control can be achieved. While the use of anti-TNF-α antibodies can achieve PASI75# in a majority of patients, anti-IL17A antibodies and anti-IL-23 antibodies result in PASI100** in approximately half of patients, highlighting the relevance of the IL-23/IL-17 pathway in psoriasis. The efficacy of biologics has been compared in network meta-analyses [20]. Depending on patient characteristics, including any comorbidities that may be present, one or the other substance class is more likely to be preferred. In Switzerland, in addition to TNF-α inhibitors and the IL12-/23 antibody ustekinumab, several active substances from the groups of anti-IL17A antibodies (secukinumab, ixekizumab) and anti-IL-23 antibodies (guselkumab, risankizumab, tildrakizumab) are currently approved [25]. And the IL17A/17F inhibitor bimekizumab has now also cleared the approval hurdles [25]. From the field of “small molecules”, the PDE-4 inhibitor apremilast is an approved oral drug. In addition, deucravacitinib (oral TYK2-i), roflumilast (topical PDE-4-i), and tapinarof (topical AHR agonist) are currently under clinical investigation [21–23]. |
| # PASI75 = an improvement in PASI (Psoriasis Area and Severity Index) of at least 75%. ** PASI 100 = a 100% improvement in PASI (Psoriasis Area and Severity Index). |
CD4+ Th cells are known to secrete various cytokines. The importance of these messenger substances in psoriasis pathogenesis is confirmed by the very good therapeutic response with targeted neutralization of individual cytokines of the immune system. First, it was found that blocking TNF-α helped to alleviate psoriasis [32,33]. Ustekinumab, a biologic targeting the common p40 subunit of IL-12 and IL-23, was later approved and showed good efficacy in psoriasis [1]. Monoclonal antibodies that selectively target the p19 subunit of IL-23 (guselkumab, risankizumab, tildrakizumab) have been shown to be even more effective (box). Biologics targeting IL-17A (secukinumab, ixekizumab) or dual inhibition of IL-17A and IL17F (bimekizumab) are also highly effective (see box).
In addition to the various classes of biologics (TNFα-, IL-17-, IL-12/23- or IL-23- antagonists) and conventional system therapeutics (ciclosporin A, methotrexate, acitretin or fumaric acid esters), small molecule agents also play a role in the drug therapy of psoriasis. Apremilast is an oral PDE-4 (phosphodiesterase-4) inhibitor approved for the treatment of psoriasis, among other indications. The anti-inflammatory effects of apremilast are brought about by an increase in intracellular cAMP levels. Furthermore, Roflumilast, a topically applicable PDE-4 inhibitor, is currently being tested in clinical trials [34]. This also applies to other representatives of the “small molecules ” (see box) .
Literature:
- van de Kerkhof PC: From Empirical to Pathogenesis-Based Treatments for Psoriasis. J Invest Dermatol 2022; 142(7): 1778-1785.
- Di Meglio P, Villanova F, Nestle FO: Psoriasis. Cold Spring Harb Perspect Med 2014; 4(8): 1-30.
- Prinz JC: Human Leukocyte Antigen-Class I Alleles and the Autoreactive T Cell Response in Psoriasis Pathogenesis. Front Immunol 2018; 9: 954.
- Schäkel K, Schön M, Ghoreschi K: Pathogenesis of psoriasis vulgaris. Der Hautarzt 2016; 67(6): 422–431.
- Di Cesare A, Di Meglio P, Nestle FO: The IL-23/Th17 axis in the immunopathogenesis of psoriasis. Journal of Investigative Dermatology 2009; 129(6): 1339–1350.
- Lynde CW, et al.: Interleukin 17A: toward a new understanding of psoriasis pathogenesis. Journal of the American Academy of Dermatology 2014; 71(1): 141–150.
- Johansen C, et al.: Inverse regulation of the nuclear factor-κB binding to the p53 and interleukin-8 κB response elements in lesional psoriatic skin. Journal of investigative Dermatology 2005; 124(6): 1284–1292.
- Eyerich S, et al.: Mutual antagonism of T cells causing psoriasis and atopic eczema.N Engl J Med 2011; 365: 231–238.
- Schneider S, Li L, Zink A: Psoriasis – Differentialdiagnosen und Therapie. Akt Rheumatol 2022; 47: 324–332.
- Greb JE, et al.: Psoriasis. Nature Reviews Disease Primers 2016; 2: 16082.
- Lowes MA, et al.: Increase in TNF-alpha and inducible nitric oxide synthase-expressing dendritic cells in psoriasis and reduction with efalizumab (anti-CD11a). Proc Natl Acad Sci U S A 2005; 102: 19057–19062.
- Conrad C, et al.: Alpha1beta1 integrin is crucial for accumulation of epidermal T cells and the development of psoriasis. Nat Med 2007; 13: 836–842.
- Bovenschen HJ, Seyger MM, van de Kerkhof PC: Plaque psoriasis vs. atopic dermatitis and lichen planus: a comparison for lesional T-cell subsets, epidermal proliferation and differentiation. Br J Dermatol 2005; 153: 72–78.
- Clark RA: Resident memory T cells in human health and disease. Sci Transl Med. 2015 Jan 7; 7(269): 269rv1.
- Cheuk S, et al. Epidermal Th22 and Tc17 cells form a localized disease memory in clinically healed psoriasis. J Immunol 2014; 192: 3111-3120.
- Benezeder T, Wolf P: Resolution of plaque-type psoriasis: what is left behind (and reinitiates the disease). Semin Immunopathol 2019; 41: 633-644.
- Prinz JC: Melanocytes: Target Cells of an HLA-C*06:02-Restricted Autoimmune Response in Psoriasis. J Invest Dermatol 2017; 137(10): 2053-2058.
- Arakawa A, et al: Melanocyte antigen triggers autoimmunity in human psoriasis. J Exp Med 2015; 212(13): 2203-2212.
- Hawkes JE, Chan TC, Krueger JG: Psoriasis pathogenesis and the development of novel targeted immune therapies. J Allergy Clin Immunol 2017; 140(3): 645-653.
- Armstrong AW, et al.: Comparison of biologics and oral treatments for plaque psoriasis: a meta-analysis. JAMA Dermatol 2020; 156: 258–269.
- Papp K, et al.: Phase 2 trial of selective tyrosine kinase 2 inhibition in psoriasis. N Engl J Med. 2018; 379: 1313–1321.
- Lebwohl MG, et al.: Trial of roflumilast cream for chronic plaque psoriasis. N Engl J Med. 2020; 383: 229–239.
- Robbins K, et al: Phase 2, randomized dose-finding study of tapinarof (GSK2894512 cream) for the treatment of plaque psoriasis. J Am Acad Dermatol 2019; 80: 714-772.
- Mohd Noor AA, Azlan M, Mohd Redzwan N: Orchestrated Cytokines Mediated by Biologics in Psoriasis and Its Mechanisms of Action. Biomedicines. 2022 Feb 20;10(2): 498. www.mdpi.com/2227-9059/10/2/498,(last accessed Mar 15, 3023).
- Drug Information, www.swissmedicinfo.ch,(last accessed Mar. 15, 2023).
- Ellis CN, et al.: Cyclosporine improves psoriasis in a double-blind study. JAMA 1986; 256: 3110-3116
- Griffiths CE, et al: Clearance of psoriasis with low dose cyclosporine. Br Med J (Clin Res Ed) 1986; 293: 731-732.
- Abrams JR, et al: Blockade of T lymphocyte costimulation with cytotoxic T lymphocyte-associated antigen 4-immunoglobulin (CTLA4Ig) reverses the cellular pathology of psoriatic plaques, including the activation of keratinocytes, dendritic cells, and endothelial cells. J Exp Med 2000; 192: 681-694.
- Nicolas JF, et al.: CD4 antibody treatment of severe psoriasis. Lancet 1991; 338: 321
- Wrone-Smith T, Nickoloff BJ: Dermal injection of immunocytes induces psoriasis. J Clin Invest. 1996; 98: 1878-1887.
- Nickoloff BJ, Wrone-Smith T: Injection of pre-psoriatic skin with CD4+ T cells induces psoriasis. Am J Pathol 1999; 155: 145-158.
- Zaba LC, et al. : Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses [published correction in J Exp Med 2008;205:1941]. J Exp Med. 2007; 204: 3183-3194.
- Zaba LC, et al. Effective treatment of psoriasis with etanercept is linked to suppression of IL-17 signalling, not immediate response TNF genes. J Allergy Clin Immunol. 2009; 124: 1022-1030.E395
- Lebwohl MG, et al.: Trial of roflumilast cream for chronic plaque psoriasis. N Engl J Med. 2020; 383: 229–239.
DERMATOLOGIE PRAXIS 2023; 33(2): 20–22