An overarching principle for therapy selection in psoriatic arthritis is shared decision making with patients. The GRAPPA recommendations propose an individualized treatment approach that takes into account the predominant disease domains according to the Caspar classification, but also incorporates comorbidities and patient preferences.
When psoriasis patients report joint pain (“Pain”), stiffness (“Stiffness”), and back pain (“Axial involvement”) that improves with physical activity, dermatologists should think of psoriatic arthritis [1]. In up to one third of patients with plaque psoriasis, inflammatory involvement of entheses and joints develops in the course of the disease, which belong to the spondylarthritides (SpA) and are summarized under the collective term psoriatic arthritis (PsA) [2]. PsA is classified as peripheral SpA, whereas axial SpA includes ankylosing spondylitis [3]. Swelling and tenderness around the joints are characteristic of PsA. In addition to radiating thickening of individual fingers or toes (dactylitis), pain may occur in the area of tendon insertions (enthesitis) [4]. The Caspar classification (“Classification Criteria for Psoriatic Arthritis”) is helpful in establishing the diagnosis. This diagnostic tool detects sacroilitis, enthesitis, dactylitis and nail infestation, among others [10]. This classification is the basis for GRAPPA* treatment recommendations.
* GRAPPA=Group for Research and Assessment of Psoriasis and Psoriatic Arthritis
Domain-specific treatment approach recommended
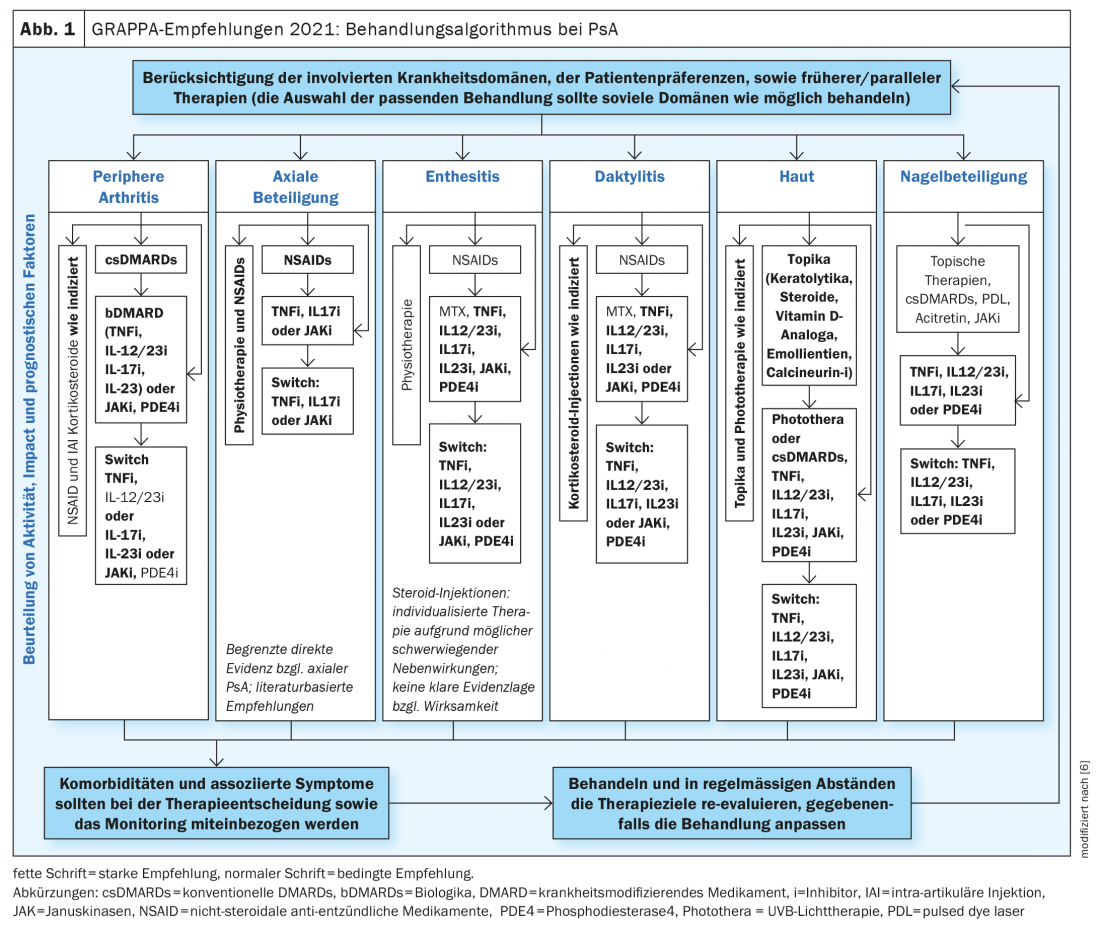
There is a wide range of treatment options for PsA today. Biologics (bDMARDs) or “small molecules” (tsDMARDs) are used when there is an insufficient response to conventional systemic therapeutics (csDMARDs). Joseph Merola, MD, dermatologist and rheumatologist, Harvard Medical School, Boston (USA), spoke at the EADV annual meeting on criteria for selecting the most appropriate individualized treatment for patients with PsA [5]. According to current expert assessment, including the GRAPPA recommendations on diagnosis and treatment of Pso and PsoA, a domain-specific approach is advocated (Fig. 1), taking into account which of the following disease domains is predominant: peripheral artrhitis, axial involvement, enthesistis, dactylitis, skin psoriasis, nail involvement [5,6].
|
The role of proinflammatory cytokines in PsA pathogenesis. The majority of PsA patients initially present with psoriatic skin symptoms before joint involvement is diagnosed after an average of ten years [7]. The typical age of manifestation of PsA is 35-50 years [8]. Pathophysiologically, predisposing genetic factors result in aberrant immunologic processes, leading to overexpression of proinflammatory cytokines (e.g., TNF, IL-17, IL-23, and IL-6). These cause cytokine-mediated invasion of a wide variety of immune cells at the site of inflammation, which in turn secrete further cytokines and thus amplify and maintain the inflammatory response [7,8]. In connection with these processes, there is an increased inflammatory reaction in the inner layer of the joint capsules (synovitis), which leads to damage of the articular cartilage as well as bone erosion [8]. The various cytokines secreted in the course of the inflammatory response play a role in the different disease manifestations of PsA and represent potential therapeutic targets. The therapeutic goal is primarily to reduce radiographic progression and inflammation. Severe joint involvement argues for early use of DMARDs. Thanks to state-of-the-art therapies, remission is now an achievable treatment goal in PsA [9]. |
For peripheral arthritis, most available drugs are similarly effective. Accordingly, biologics, as well as JAK inhibitors and phosphodiesterase-4 (PDE-4) inhibitors, may be considered in cases of inadequate response to conventional disease-modifying drugs (csDMARDs). In particular, there is a good evidence base for TNF-alpha inhibitors as well as several interleukin inhibitors (IL-12/23-i, IL-17-i, and IL-23-i).
In PsA patients with axial arthritis, TNF-alpha inhibitors and IL-17 inhibitors, as well as JAK inhibitors, have been most effective, in addition to nonsteroidal anti-inflammatory drugs (NSAIDs) and physical therapy. According to GRAPPA, the data situation is currently insufficient for IL-12/23 and IL-23 inhibitors.
In PsA patients with enthesitis and dactylitis, the best evidence is for TNF-alpha-i as well as IL-12/23-i, IL-17-i, and IL-23-i.
In nail psoriasis, the best results are achieved with TNF-alpha inhibitors, IL-12/23-i, IL-17-i and IL-23-i, and PDE-4 inhibitors.
Comorbidities such as inflammatory bowel disease (IBD) or uveitis must also be included in the treatment decision. If a PsA patient has IBD as a concomitant disease, the use of TNF-alpha inhibitors (except etanercept), IL-12/23-i, and JAK-i is preferred. For comorbid uveitis, TNF-alpha inhibitors (with the exception of etanercept) are the first line of treatment. Last but not least, in addition to the predominant disease domains and comorbidities, patient preferences regarding route of administration (oral vs. subcutaneous) and treatment interval must be considered when selecting the appropriate system therapy.
Congress: EADV Annual Meeting
Literature:
- Gottlieb A, et al: Psoriatic arthritis for dermatologists. J Dermatolog Treat 2020; 31(7): 662-679.
- Sticherling M: Use of secukinumab in patients with psoriasis and psoriatic arthritis – results of a non-interventional study under real-life conditions (SERENA study). Compass Autoimmune 2021; 3: 118-120.
- Proft F, Poddubnyy D: Ankylosing spondylitis and axial spondyloarthritis: recent insights and impact of new classification criteria. Ther Adv Musculoskelet Dis 2018; 10(5-6): 129-139.
- Borst C: Psoriatic arthritis. close up 2020; 19: 146-151.
- Merola JF: Personalized Care in Psoriatic Arthritis, Joseph Merola, MD, EADV Annual Meeting, EADV Annual Meeting, 9/29-21-10/21.
- Coates LC et al. The Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) Treatment Recommendations 2021, eEULAR 2021, Abstract OP0229, http://dx.doi.org/10.1136/annrheumdis-2021-eular.4091
- Ritchlin CT, Colbert RA, Gladman DD: Psoriatic arthritis. N Engl J Med 2017; 376(10): 957-970.
- Veale DJ, Fearon U: The pathogenesis of psoriatic arthritis. Lancet. 2018; 391(10136): 2273-2284.
- Tucker LJ, Ye W, Coates LC: New approaches to the management of psoriatic arthritis: can we target treatment? Karger Compass Autoimmune 2019; 1: 8-16.
- Tillett W, et al: The ClASsification for Psoriatic ARthritis (CASPAR) criteria – a retrospective feasibility, sensitivity, and specificity study. J Rheumatol 2012; 39(1): 154-156.
- Creakyjoints.org, https://creakyjoints.org (last accessed Nov. 19, 2021).
DERMATOLOGIE PRAXIS 2021; 31(6): 20-21