The incidence of brain metastases increases in proportion to the prolonged survival, significantly worsening the prognosis. Intensity-modulated radiotherapy techniques can spare the hippocampus.
Advances in primary tumor therapy have increased extracerebral local tumor control and patient life expectancy. Proportionally to prolonged survival, the incidence of brain metastases increases. They represent the most common malignant cerebral tumors, and their occurrence critically worsens the prognosis.
An assessment of prognosis, tumor burden intra- and extracranially, and the patient’s general condition are required to select an individualized metastatic treatment. In many cases, multiple symptomatic metastases leave only improvement or elimination of neurologic symptoms as the primary treatment goal. This fact should be taken into account by a correspondingly short duration of therapy with rapid onset of action in order to ensure the best possible quality of life. Depending on the overall clinical situation, a partly significant improvement in quality of life can be achieved in about 80%. However, the indication for therapy becomes increasingly relative as the underlying disease progresses and the general condition deteriorates rapidly. If the prognosis is poor and the life expectancy is only a few weeks, radiotherapy can be dispensed with in favor of supportive measures alone in order to preserve the quality of life [1]. On the other hand, in the case of single or a few lesions and controlled extracerebral disease and thus favorable prognosis, permanent local control should be sought by intensified therapy.
Radiation therapy treatment options for brain metastases include whole-brain irradiation with or without focal dose escalation, as well as stereotactic techniques including intensity modulation (IMRT) and radiosurgery. The latter can be the sole form of therapy, but can also be combined with whole-brain radiation or microsurgical resection. These techniques also offer renewed treatment options after whole-brain radiation and new or progressive brain metastases have occurred.
Therapy methods
Whole brain irradiation: patients with multiple metastases who are not candidates for surgery or stereotactic radiation usually receive palliative whole brain irradiation as part of their treatment. Unselected collectives with primary tumors of different histologies benefit only moderately in terms of survival. The median survival is between 3 and 6 months, and only about 10 to 15% of patients live longer than one year.
The entire contents of the skull are treated with high-energy X-rays or gamma rays via lateral fields (Fig. 1) . Doses of 30 Gy in 10 or 20 Gy in 5 fractions are equivalent [2].

The clinical symptoms of acute radiation effects on the brain usually correspond to those of intracranial pressure increase. Headaches, fasting vomiting, and even impaired consciousness may occur. Subacute damage is usually nonspecific, such as slowing, impaired concentration/memory, and somnolence. The clinical spectrum of late toxicity ranges from discrete neuropsychological deficits to neurological deficits and cerebral necrosis. In the case of whole-brain irradiation, higher single doses in particular lead to the development of symptomatic late damage. A particularly sensitive region appears to be the hippocampus, whose stem cells are responsible for neurogenesis and maintenance of cognitive abilities. Sparing this structure can significantly reduce intellectual losses after whole-brain irradiation [3]. Modern techniques such as IMRT (intensity-modulated radiotherapy) allow this to be done by finely modulating the dose distribution. For example, targeted underdosing can be performed in critical structures, such as the hippocampus (Figs. 2A and 2B). Focused irradiations of smaller volumes, e.g., in the form of radiosurgery, are better tolerated [4,5].

Whole brain irradiation in combination with surgery or radiosurgery: metastatic resection can result in rapid relief of neurologic symptoms, intracranial pressure, and steroid requirements, especially in large lesions with extensive edema. Adjuvant whole-brain radiation following local therapy, whether resection or radiosurgery, is intended to destroy microscopic locoregional or distant cerebral residuals. Patchell [6] also demonstrated that despite MR-tomographically proven complete resection, postoperative irradiation highly significantly improved local control. The cerebral recurrence rate was reduced locally from 46% to 10% and distantly from 70% to 18%. Although this did not result in a survival benefit, significantly fewer patients died from neurological sequelae. The same results were obtained in a randomized phase III EORTC trial (22952-26001). The patients were in good general condition and suffered from solid tumors. After resection or radiosurgery of 1 to 3 cerebral metastases, randomization to whole-brain irradiation with 30.0 Gy in 10 fractions or imaging control alone was performed. Although the patients recruited had stable systemic disease and thus would have been most likely to benefit from whole-brain irradiation, again there was no survival benefit [7]. A meta-analysis [8] also found no survival benefit after combination therapy in the most prognostically favorable group of patients with 1 to 4 metastases, a KPS of 70 or higher, and an age of 50 years or younger.
Large-volume radiation can limit quality of life and cognitive abilities. In the EORTC study [9] and others, more pronounced fatigue and mildly reduced physical and cognitive functioning were observed as treatment sequelae. Therefore, after complete resection of a limited number of metastases, adjuvant whole-brain radiation should be indicated very restrictively.
Stereotactic radiation techniques: Stereotactic radiosurgery and fractionated stereotactic irradiation have long been used successfully. These are highly precise techniques that use stereotactic coordinates to apply extremely focused ionizing beams into a precisely defined volume of tissue. The goal is to destroy the proliferating tumor tissue while optimally sparing the adjacent nervous structures. Indispensable for this is a 3-dimensional delineation of the target volume and risk structures based on high-resolution stereotactic CT or MRI datasets. Brain metastases are often characterized by optimal image morphologic detection and delineation on CT and MRI, small volume, and spherical shape. They thus offer good conditions for the best possible feasibility and tolerability of radiosurgery. An extremely steep dose fall-off to the brain parenchyma and perfect matching of the prescribed dose to the target volume contour (conformation) result in sparing of the healthy brain. This allows the application of very high doses (18 to 25 Gy), which enable local control rates of about 80 to 90% with few complications even in so-called radioresistant histologies [10].
However, as lesion size increases, the risk of complications in healthy brain tissue increases [11]. This results in a limitation of one-time radiosurgery to a maximum lesion diameter of 3 cm (approximately 15 ml volume). Larger metastases can be treated equivalently by so-called fractionated radiosurgery in three fractions of 9.0 Gy [12].
Meanwhile, modern planning systems in combination with dynamic micromultileaf collimators also allow simultaneous radiosurgical therapy of multiple metastases. This allows local ablative doses to be applied to individual lesions with better tolerance of small volume irradiation. Technically, simultaneous dose increases in individual macroscopic metastases (Fig. 3) are also possible, in addition to simultaneous sparing of the hippocampi. The limitation to a maximum of 3 metastases, which was previously considered reasonable, is now no longer valid. Patients with up to more than 10 metastases also benefit from radiosurgery [13]. The determining factor for toxicity seems to be rather the total volume of lesions.

Acute reactions due to edema may occur in approximately 10% to 40% within 2 weeks and may manifest as headache, nausea with vomiting, seizures, or worsening of preexisting neurologic deficits. These reactions are generally reversible by steroids. Chronic complications include hemorrhage and radionecrosis (1% to 17%).
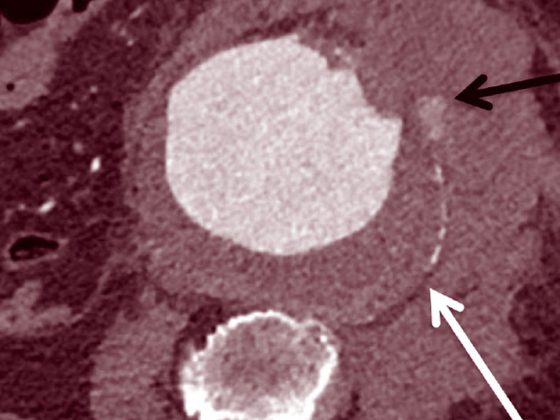
A transient increase in size of the irradiated lesion with more extensive edema and mass effect is occasionally found after 1 to 3 months and cannot be distinguished from true tumor progression. In patients who often have few or no symptoms, imaging should be performed first before invasive therapeutic measures (Fig. 4).

Stereotactic radiation after resection: After metastatic resection, stereotactic single-treatment or fractionated radiosurgery of the metastatic bed can improve local control. However, survival is not expected to be prolonged any more than by postoperative whole-brain irradiation. The higher individual doses increase the risk of radionecrosis compared with whole-brain irradiation. In addition, an increased incidence of regional leptomeningeal seeding was seen [14].
Re-irradiation: In the treatment of recurrent metastases, re-irradiation one-time therapy is again able to achieve appreciable improvements in survival and neurological quality of life. Local control rates of over 80% and median survival of up to 10 months are achievable [15]. Reirradiation of the same metastases is also possible with moderate risk of side effects [16]. These measures can avoid the need for whole-brain irradiation over an extended period of time.
Take-Home Messages
- Whole-brain radiation still has its place in cases of multiple metastases and poor prognosis.
- Intensity modulated techniques allow reduction of cognitive impairment by hippocampal sparing.
- After resection, stereotactic irradiation of the metastatic bed may improve local control.
- For small, even multiple metastases, stereotactic radiosurgery is an effective and well-tolerated therapy even in the first-line setting.
- Fractional radiosurgery can be used to treat larger lesions to reduce toxicity.
- In case of recurrence (local or distant), stereotactic radiosurgery can also be repeated.
Literature:
- Mulvenna P, et al: Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): results from a phase 3, non-inferiority, randomised trial. Lancet 2016; 388(10055): 2004-2014.
- Tsao M, et al: A meta-analysis evaluating stereotactic radiosurgery, whole-brain radiotherapy, or both for patients presenting with a limited number of brain metastases. Cancer 2012; 118(9): 2486-2493.
- Gondi V, et al: Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol 2014; 32(34): 3810-3816.
- DeAngelis LM, et al: Radiation-induced dementia in patients cured of brain metastases. Neurology 1989; 39: 789-796.
- Patel KR, et al: Intracranial control and radiographic changes with adjuvant radiation therapy for resected brain metastases: whole brain radiotherapy versus stereotactic radiosurgery alone. J Neurooncol 2014; 120: 657-663.
- Patchell RA, et al: A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 1990; 322(8): 494-500.
- Kocher M, et al: Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol 2011; 29: 134-141.
- Sahgal A, et al: Phase 3 trials of stereotactic radiosurgery with or without whole-brain radiationtherapy for 1 to 4 brain metastases: individual patient data meta-analysis. Int J Radiat Oncol Biol Phys 2015; 91(4): 710-717.
- Soffietti R et al: A European Organisation for Research and Treatment of Cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: quality-of-life results. J Clin Oncol 2013; 31: 65-72.
- Manon R, et al: Phase II trial of radiosurgery for one to three newly diagnosed brain metastases from renal cell carcinoma, melanoma, and sarcoma: an Eastern Cooperative Oncology Group study (E 6397). J Clin Oncol 2005; 23(34): 8870-8876.
- Minniti G et al: Stereotactic radiosurgery for brain metastases: analysis of outcome and risk of brain radionecrosis. Radiat Oncol 2011; 6: 48-56.
- Minniti G, et al: Single-fraction versus multifraction (3 x 9 Gy) stereotactic radiosurgery for large (>2 cm) brain metastases: A comparative analysis of local control and risk of radiation-induced brain necrosis. Int J Radiation Oncol Biol Phys 2016; 95(4): 1142-1148.
- Yamamoto M, et al: Stereotactic radiosurgery for patients with multiple brain metastases: a case-matched study comparing treatment results for patients with 2-9 versus 10 or more tumors. J Neurosurg 2014; 121 (Suppl. 2): 16-25.
- Hsieh J, et al: Tumor progression in patients receiving adjuvant whole-brain radiotherapy vs localized radiotherapy after surgical resection of brain metastases. Neurosurgery 2015; 76(4): 411-420.
- Shultz DB, et al: Repeat courses of stereotactic radiosurgery (SRS), deferring whole-brain irradiation, for new brain metastases after initial SRS. Int J Radiat Oncol Biol Phys 2015; 92(5): 993-999.
- McKay WH, et al: Repeat stereotactic radiosurgery as salvage therapy for locally recurrent brain metastases previously treated with radiosurgery. J Neurosurg 2017; 127: 148-156.
InFo ONCOLOGY & HEMATOLOGY 2019; 7(2-3): 16-19.