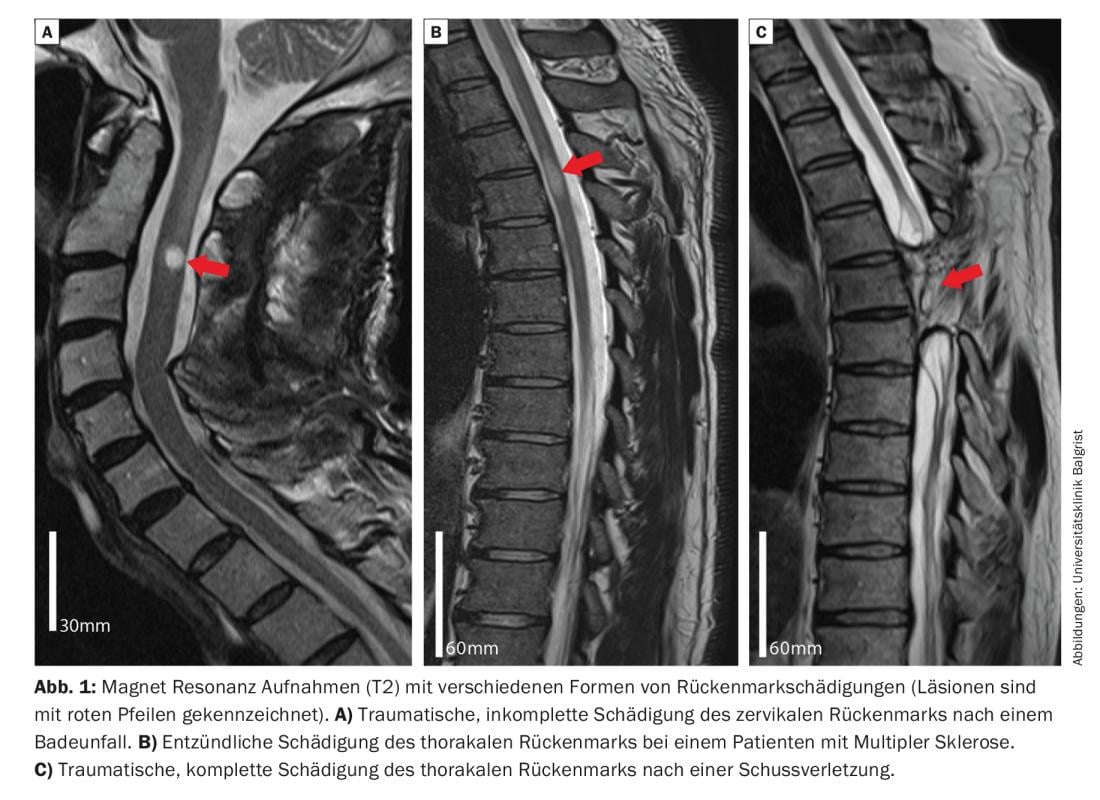
People with lesions below the cervical spinal cord segments have spastic or flaccid paraplegic paraplegia. Restrictions in walking ability are considered serious by affected patients and are the focus of this educational article.
Damage to the spinal cord can result from traumatic, inflammatory, or even vascular causes and is often associated with severe, often lifelong, persistent limitations in motor, sensory, and autonomic functions. Approximately half of spinal cord injuries are traumatic in origin [1], with 50% of traumatic injuries to the myelon being incomplete and preserving sensory and/or motor functions below the lesion [2]. Depending on the extent and localization of the spinal damage, different ascending and descending nerve tracts are injured (Fig. 1). This leads to various functional deficits, which can range from mild sensory disturbances and weakness in individual muscle groups to inability to walk and complete loss of urinary bladder, bowel and sexual function. Traumatic injuries most commonly affect the cervical spinal cord, which can result in impaired breathing and deficits in the upper and lower extremities (quadriplegia) [3]. People with lesions below the cervical spinal cord segments have spastic (thoracic or lumbar lesion) or flaccid (damage to conus/cauda equina) paraplegic paralysis. Limitations in walking ability are considered severe by affected patients [4] and are the focus of this article.
Mechanisms of spontaneous recovery after spinal cord injury.
In the central nervous system there is only a limited capacity for regeneration processes after injuries. Thus, severed nerve fibers cannot regrow over long distances to restore original nerve connections. Nevertheless, many patients show spontaneous functional recovery after spinal cord injury. Research results from animal experimental studies showed that different processes and mechanisms are responsible for functional recovery in the acute, subacute, and chronic phases after spinal injury. These include decreasing edema and inflammatory processes at the site of injury, remittent neuronal excitotoxicity in perilesional neural tissue [5,6], partial re-myelination of demyelinated nerve fibers [7], and various forms of neuronal plasticity [8]. Lesion-induced plastic adaptations of the central nervous system range from changes at the molecular level (e.g., upregulation of receptors on motor neurons and interneurons [9]) to structural modifications of neuronal networks [10,11]. Uninjured nerve fibers can thereby sprout into spinal regions denervated by the damage and restore functions (compensatory sprouting of intact axons) [12]. Injured nerve fibers severed by the lesion show limited local growth (regenerative sprouting of injured axons) and may, for example, re-project in the sense of redirection to propriospinal interneurons, which in turn allow signal transmission around the lesion site [13]. Such structural adaptations within the central nervous system have been observed in animal models in functionally distinct, neural systems and networks (e.g., in the cortico-spinal, bulbo-spinal, and proprio-spinal systems) and have been associated with recovery of gross and fine motor functions after spinal cord damage [14].
Neurobiological effects of training
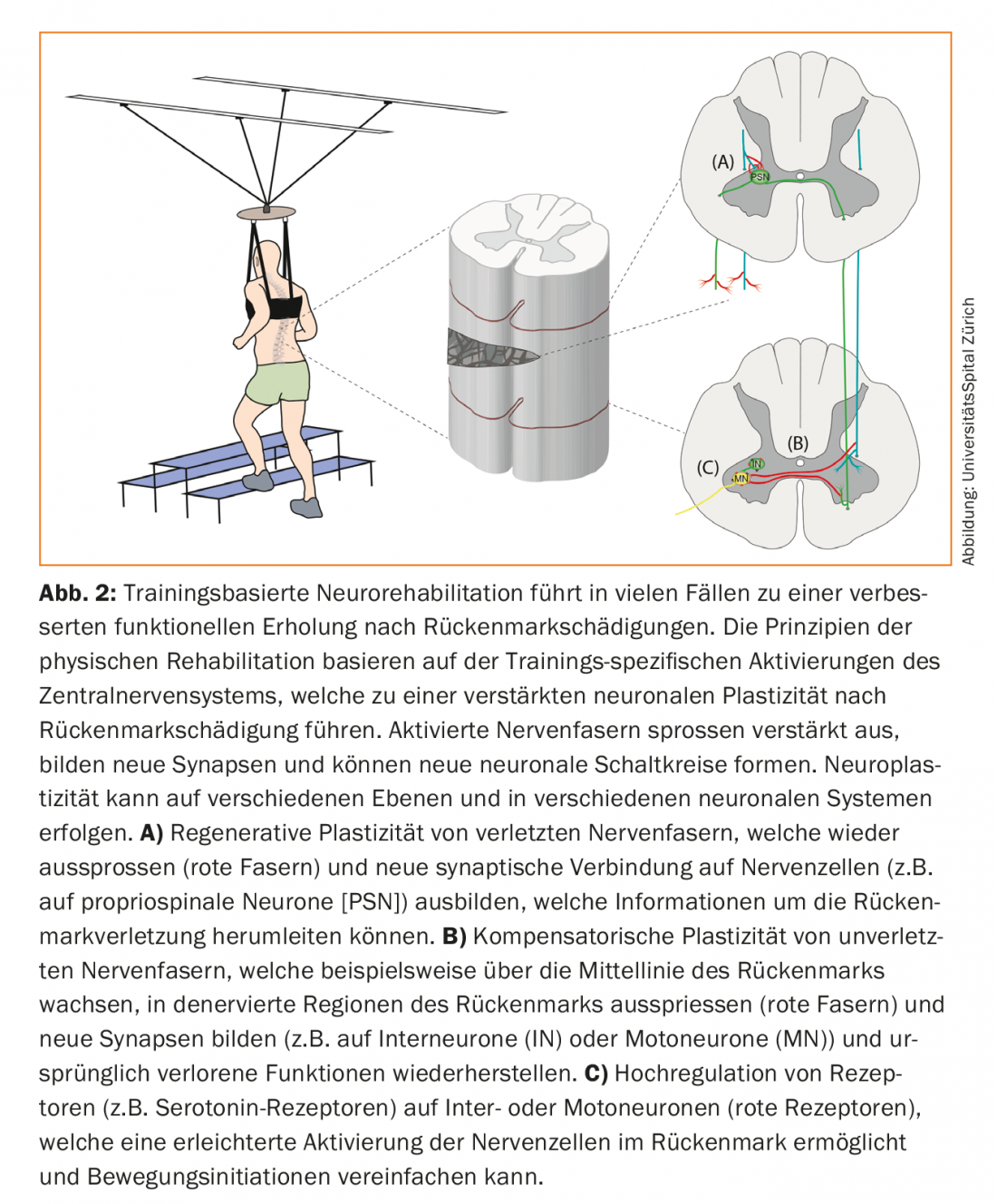
One goal of current research is to promote and enhance the described spontaneous mechanisms of functional recovery after spinal cord injury through therapies. Despite promising pre-clinical trial results, no effective pharmacological treatments are currently available for patients with spinal cord injury. Physical training, on the other hand, is an integral part of current neurorehabilitation. Studies have demonstrated significant improvements in motor function with exercise in patients with incomplete paraplegia [15]. The principles of exercise-based neurorehabilitation are based on the activation of neural circuits above as well as below the spinal cord injury. Spinal and supraspinal neurons can reorganize and interconnect through training [16] (Fig. 2). Training-induced activation of neuronal networks enhances spontaneous sprouting of injured and uninjured nerve fibers. In this process, purpose-bound projections are consolidated, while unused, redundant neural connections are dismantled (neuronal pruning) [17]. The molecular and synaptic mechanisms underlying the training effects probably correspond to those of learning theory: simultaneous activity of associated neuronal systems is stabilized, whereas nonsynchronized activity leads to the dissolution of neural connections (Hebb’s learning rule) [18]. In addition, physical training has a positive effect on the patient’s entire musculoskeletal and cardiovascular systems. The intensity, optimal period and type of training, and the role of patient motivation are the subject of current research [19].
Clinical focus in the rehabilitation of patients with spinal cord injury
The primary rehabilitation focus is regaining independence. Urinary bladder and bowel management that is as independent as possible is a fundamental goal of rehabilitation. Depending on the severity and type of spinal injury, the physical rehabilitation plan varies. In patients with high-cervical spinal lesions, the focus is on restoring spontaneous breathing and independence (e.g., with tongue-based control mechanisms). While deeper cervical lesions are trained to control arm/hand movements, thoracic and lumbar spinal lesions focus on mobility and locomotion. In this context, the field of physical rehabilitation is becoming increasingly interdisciplinary: therapists are often supported by robots or highly instrumentalized training apparatuses that enable intensive, individualized training (e.g., via weight relief systems, dynamic force resistance, etc.) and thus optimize functional recovery [20,21].
New therapeutic approaches in the field of physical rehabilitation aim, among other things, at increasing patient motivation during training: virtual reality (VR)-based training programs project realistic, varied everyday situations into monotonous laboratories and training rooms and can thus reinforce the efficiency of training by increasing motivation [22,23]. Furthermore, innovative instrumentalized training apparatuses have been developed, which allow targeted leg exercise adapted to the patient’s particular limitation. Patients with severe walking impairments can, for example, be trained in the gait robot (Lokomat®; Hocoma AG, Switzerland). Here, an exoskeleton and a dynamic weight-relief system support the patient during walking and enable individually adapted, intensive gait training. A new generation of transparent weight-bearing systems (e.g. The FLOAT®, Switzerland) enables locomotion training in patients with moderate to severe walking disabilities. These dynamic systems use online feedback to permanently adjust weight relief, providing multidimensional gait and balance training without resistance to movement (but including fall protection). This allows intensive, secured training of movements relevant to everyday life and complex movements (e.g. walking around curves, climbing stairs, overcoming obstacles), which can lead to improved functional recovery compared to conventional training methods (e.g. treadmill training) [24]. Another instrumentalized training device is the Grail System (MotekForce Link; NL): it provides aggravated, game-like gait training for patients with mild to moderate gait disorders. The system includes direct performance feedback for patients and a system for inducing unexpected disturbances (3D movements of the treadmill) during walking [25].
New therapeutic approaches
New rehabilitative strategies after spinal cord injury include electrical excitation of spinal neurons below the lesion via epidural [26] or transcutaneous [27] stimulators. Extrinsic excitation of spinal neurons below the spinal cord injury is thought to allow peripheral afferents and residual supraspinal signals to activate the spinal cord more readily. This facilitated activation of neurons below the injury may improve postural stability, walking ability, and bladder function in patients with paraplegia [26,27]. The optimal stimulation algorithms and appropriate target population for electrical spinal cord stimulation are currently under investigation. Other therapies seek to stimulate nerve growth and neuroregeneration. Preclinical studies in rodents and primates showed that neutralizing antibodies against the growth inhibitory protein Nogo-A (anti-Nogo-A antibody) localized in the myelin of the central nervous system leads to increased neuronal plasticity (including sprouting of nerve fibers, new connections between nerve fibers) and improved functional recovery after incomplete spinal cord injury [11,28]. The effect of neutralizing anti-Nogo-A antibodies on the recovery of patients with spinal cord injury is currently being evaluated in a clinical trial. Another therapeutic approach for spinal cord injury is based on the transplantation or implantation of stem cells or neural progenitor cells directly into the spinal lesion. Studies in rodents found that transplanted stem/progenitor cells can successfully differentiate into glial cells and neurons: Glial cells can thereby induce partial re-myelination and neuroprotection, while newly differentiated neurons can outgrow, establish new synaptic connections, and lead to improved functional recovery [29,30]. Despite numerous, pre-clinical data, the effects of cell transplantation in humans are still controversial and should be investigated in detail in larger, controlled studies [31].
Take-Home Messages
- In many cases, damage to the spinal cord leads to lifelong persistent functional disorders such as paralysis, sensory disturbances and urinary bladder, bowel and sexual dysfunction.
- Physical rehabilitation is currently the only established therapy for patients with spinal cord injury. The activation of neural systems during training leads to neural plasticity, which stimulates the natural recovery process.
- New instrumentalized training devices provide personalized, intensive gait training for patients with varying degrees of gait impairment. Training conditions suitable for everyday use and functional feedback systems promote patient motivation.
- New therapeutic approaches such as electrical stimulation of the spinal cord, pharmacological promotion of neuronal regeneration or stem cell transplantation have already achieved promising results and may be used in the future as additional therapeutic options for patients with spinal cord injury.
Literature:
- Gupta A, Taly AB, et al: Non-traumatic spinal cord lesions: epidemiology, complications, neurological and functional outcome of rehabilitation. Spinal Cord 2009; 47(4): 307-311.
- Wyndaele M, Wyndaele JJ: Incidence, prevalence and epidemiology of spinal cord injury: what learns a worldwide literature survey? Spinal Cord 2006; 44(9): 523-529.
- McKinley W, Santos K, et al: Incidence and outcomes of spinal cord injury clinical syndromes. J Spinal Cord Med 2007; 30(3): 215-224.
- Simpson LA, Eng JJ, et al: Spinal Cord Injury Rehabilitation Evidence Scire Research T. The health and life priorities of individuals with spinal cord injury: a systematic review. J Neurotrauma 2012; 29(8): 1548-1555.
- Bunge RP, Puckett WR, et al: Observations on the pathology of human spinal cord injury. A review and classification of 22 new cases with details from a case of chronic cord compression with extensive focal demyelination. Adv Neurol 1993; 59: 75-89.
- Norenberg MD, Smith J, Marcillo A: The pathology of human spinal cord injury: defining the problems. J Neurotrauma 2004; 21(4): 429-440.
- Bartus K, Galino J, et al. Neuregulin-1 controls an endogenous repair mechanism after spinal cord injury. Brain 2016; 139(Pt 5): 1394-1416.
- Filli L, Schwab ME: Structural and functional reorganization of propriospinal connections promotes functional recovery after spinal cord injury. Neural Regen Res 2015; 10(4): 509-513.
- Murray KC, Nakae A, et al: Recovery of motoneuron and locomotor function after spinal cord injury depends on constitutive activity in 5-HT2C receptors. Nat Med 2010; 16(6): 694-700.
- Asboth L, Friedli L, et al: Cortico-reticulo-spinal circuit reorganization enables functional recovery after severe spinal cord contusion. Nat Neurosci 2018; 21(4): 576-588.
- Wahl AS, Buchler U, et al: Optogenetically stimulating intact rat corticospinal tract post-stroke restores motor control through regionalized functional circuit formation. Nat Commun 2017; 8(1): 1187.
- Zorner B, Bachmann LC, Filli L, et al: Chasing central. nervous system plasticity: the brainstem’s contribution to locomotor recovery in rats with spinal cord injury. Brain 2014; 137(Pt 6): 1716-1732.
- Filli L, Engmann AK, Zorner B, et al: Bridging the gap: a reticulo-propriospinal detour bypassing an incomplete spinal cord injury. J Neurosci 2014; 34(40): 13399-13410.
- Raineteau O, Schwab ME: Plasticity of motor systems after incomplete spinal cord injury. Nat Rev Neurosci 2001; 2(4): 263-273.
- Harvey LA, Glinsky JV, Bowden JL: The effectiveness of 22 commonly administered physiotherapy interventions for people with spinal cord injury: a systematic review. Spinal Cord 2016; 54(11): 914-923.
- Barriere G, Leblond H, et al: Prominent role of the spinal central pattern generator in the recovery of locomotion after partial spinal cord injuries. J Neurosci 2008; 28(15): 3976-3987.
- Maier IC, Schwab ME: Sprouting, regeneration and circuit formation in the injured spinal cord: factors and activity. Philos Trans R Soc Lond B Biol Sci 2006; 361(1473): 1611-1634.
- Caporale N, Dan Y: Spike timing-dependent plasticity: a Hebbian learning rule. Annu Rev Neurosci 2008; 31: 25-46.
- Yang JF, Musselman KE: Training to achieve over ground walking after spinal cord injury: a review of who, what, when, and how. J Spinal Cord Med 2012; 35(5): 293-304.
- Alcobendas-Maestro M, Esclarin-Ruz A, et al: Lokomat robotic-assisted versus overground training within 3 to 6 months of incomplete spinal cord lesion: randomized controlled trial. Neurorehabil Neural Repair 2012; 26(9): 1058-1063.
- Fleerkotte BM, Koopman B, et al: The effect of impedance-controlled robotic gait training on walking ability and quality in individuals with chronic incomplete spinal cord injury: an explorative study. J Neuroeng Rehabil 2014; 11: 26.
- Villiger M, Liviero J et al: Home-Based Virtual Reality-Augmented Training Improves Lower Limb Muscle Strength, Balance, and Functional Mobility following Chronic Incomplete Spinal Cord Injury. Front Neurol 2017; 8: 635.
- Zimmerli L, Jacky M, et al: Increasing patient engagement during virtual reality-based motor rehabilitation. Arch Phys Med Rehabil 2013; 94(9): 1737-1746.
- Mignardot JB, Le Goff CG, et al: A multidirectional gravity-assist algorithm that enhances locomotor control in patients with stroke or spinal cord injury. Sci Transl Med 2017; 9(399).
- Biffi E, Beretta E, Cesareo A, et al: An Immersive Virtual Reality Platform to Enhance Walking Ability of Children with Acquired Brain Injuries. Methods Inf Med 2017; 56(2): 119-126.
- Harkema S, Gerasimenko Y, et al: Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet 2011; 377(9781): 1938-1947.
- Gerasimenko YP, Lu DC, et al: Noninvasive Reactivation of Motor Descending Control after Paralysis. J Neurotrauma 2015; 32(24): 1968-1980.
- Freund P, Schmidlin E, et al: Nogo-A-specific antibody treatment enhances sprouting and functional recovery after cervical lesion in adult primates. Nat Med 2006; 12(7): 790-792.
- Keirstead HS, Nistor G, et al: Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci 2005; 25(19): 4694-4705.
- Lu P, Woodruff G, Wang Y, et al: Long-distance axonal growth from human induced pluripotent stem cells after spinal cord injury. Neuron 2014; 83(4): 789-796.
- Mothe AJ, Tator CH: Advances in stem cell therapy for spinal cord injury. J Clin Invest 2012; 122(11): 3824-3834.
InFo NEUROLOGY & PSYCHIATRY 2018; 16(3): 11-15.