Not all depressed patients in remission benefit from antidepressants in terms of relapse prevention. Many patients want to discontinue their antidepressant, in part because of unwanted side effects. Currently, there are no predictors of which patient will relapse and which will not. It is possible that neurobiological biomarkers may serve as predictors. The Antidepressant Discontinuation Trial (AIDA) is investigating predictors of safe discontinuation and continues to seek patients who are in remission and wish to discontinue their antidepressant.
Relapse is a highly clinically relevant problem in depression treatment. The lifetime prevalence for relapse is very high at 60% after the first episode and an increasing risk with increasing episode number [1]. Without antidepressant medication (ADM), two-thirds of patients relapse within two years, whereas with ADM, only about one-third relapse [2]. On the one hand, this shows that the risk of relapse can be reduced by 50% (from 60% to 30%) by continuing treatment with ADM. On the other hand, two-thirds of patients benefit too little from ADM because they relapsed despite ADM or because they would not have relapsed even without ADM. One-third of patients are likely to benefit and not relapse because of ADM.
Increase motivation to continue taking antidepressants
Antidepressants are known to have side effects such as sexual dysfunction or weight gain. Because of these side effects, many patients discontinue the medication at their own request and often against medical advice [3,4] – including the third who would most likely benefit from continuing ADM. In addition, there is evidence that prolonged use of ADM leads to the development of tolerance and may artificially increase the risk of relapse after discontinuation by inducing counterregulatory processes [5]. Therefore, individualized treatment with a customized treatment recommendation could have high clinical value in the long term. If patients know that continued treatment with an ADM is necessary for them personally to prevent relapse, this could also increase motivation for medication adherence.
How can the risk of recidivism be predicted?
In order to decide whether or not a particular patient will benefit from further treatment with ADM, we need predictors that predict the course of depressive illness and distinguish between different groups of patients. Predictors may include clinical information, genetic variables, biomarkers, or a combination of these variables. In fact, however, there is very little literature and research on this topic. One predictor of relapse risk after discontinuation could be the type of response to ADM. An increased risk seems to be present in patients who respond slowly but persistently to ADM, whereas patients who respond quickly but not persistently (so-called “placebo responders”) do not have an increased risk of relapse after discontinuation [6,7]. Duration of treatment is another possible predictor, which is also referred to in the guidelines on maintenance therapy [8]. The recommendations are based on observations of the natural course of depressive episodes. Since a depressive episode usually lasts six to nine months, this is also approximately the recommended length of maintenance therapy [9]. Surprisingly, however, studies to date have not clearly demonstrated that the risk of relapse after discontinuation decreases with treatment duration [2,10–12].
Another potential predictor is the number of previous episodes. Although it has been suggested that more episodes increase the risk of relapse after discontinuation, e.g., due to the fact that the overall risk of relapse increases with the number of previous episodes, two meta-analyses on this topic reached opposite conclusions [11,12].
Biomarkers as predictors of recidivism risk.
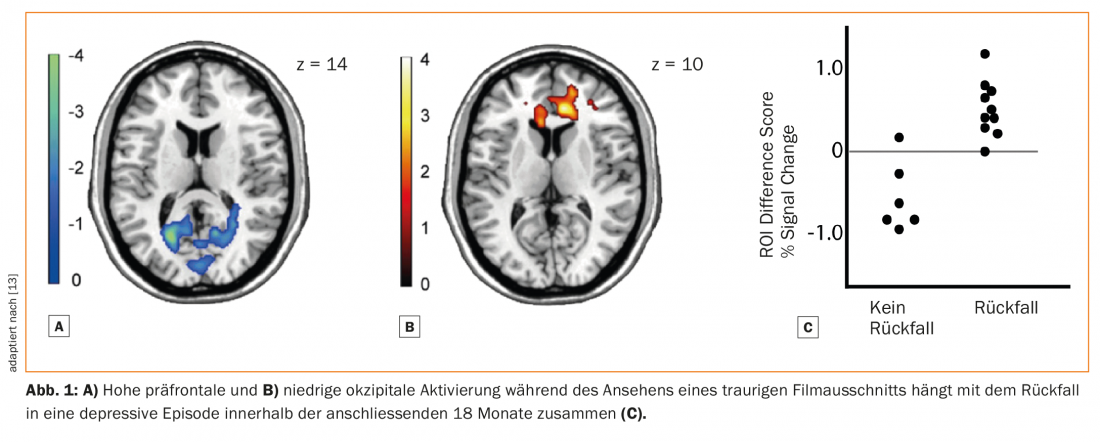
To our knowledge, no study has yet examined the potential of biomarkers as predictors of relapse risk after ADM discontinuation. Neurobiological biomarkers have only been studied in relation to overall relapse risk, independent of ADM use. For example, one study used functional MRI (fMRI) to examine brain activity while subjects watched sad movies. Decreases in occipital, relative to medial prefrontal, activity predicted relapse (Fig. 1) [13]. Another study used stimuli that elicited guilt. Here, increased connectivity between the medial prefrontal cortex and the anterior temporal lobe predicted relapse [14].
The relevant role of the medial prefrontal cortex in anhedonia, one of the core symptoms of depression, has also been confirmed in animal models. In rodents, the medial prefrontal cortex controls the dopaminergic interaction of the mesencephalon with the striatum [15]. Increased activity of the medial prefrontal cortex leads to reduced striving for reward, which is often reflected in humans as drive dysfunction.
In summary, it would be clinically useful to identify patients who would benefit from further treatment with ADM. While there are no predictors for this yet, neurobiological characteristics have great potential. To do this, it is important to examine the neurobiological effects of weaning itself, which are as yet poorly understood.

The AIDA weaning study
The aim of the Antidepressant Discontinuation Study (AIDA, www.absetzstudie.ch), funded by the Swiss National Science Foundation and the German Research Foundation, is on the one hand to find predictors for safe discontinuation of ADM, and on the other hand to investigate the effect of medication discontinuation on patients’ neurobiology and behavior. The study is ongoing at the Translational Neuromodeling Unit in cooperation with the Psychiatric University Hospital in Zurich and at the Charité in Berlin. Emotional reactivity, behavioral planning, and emotion regulation are assessed with functional imaging (fMRI and EEG). In combination with new approaches from the so-called “Computational Psychiatry” [16] these are mathematically modeled and quantified [17]. In addition, measures that have been shown to be good predictors of response to ADM will also be examined. The long-term goal is to identify clinically relevant and measurable parameters to make weaning safer.
Conclusion
Relapse prevention is an important component of successful long-term depression treatment. Although antidepressants are effective overall in preventing relapse, not all patients respond. Side effects can present significant difficulties for long-term treatments, and many patients choose not to pursue long-term drug treatment. To minimize relapse, it would be important to identify patients who can safely discontinue their medication. To date, however, no predictors have been found to predict safe discontinuation. Neurobiological biomarkers could serve as good predictors. Therefore, we are currently conducting the Antidepressant Discontinuation Study (AIDA) in Zurich and Berlin, an observational study with the aim of identifying predictors of safe discontinuation.
Literature:
- American Psychological Association: Diagnostic and statistical manual-text revision (DSM-IV-TR). APA 2000.
- Geddes JR, et al: Relapse prevention with antidepressant drug treatment in depressive disorders. A systematic review. Lancet 2003; 361(9358): 653-661.
- Olfson M, et al: Continuity of Antidepressant Treatment for Adults With Depression in the United States. Am J Psychiatry 2006; 163(1): 101-108.
- Hunot VM, et al: A Cohort Study of Adherence to Antidepressants in Primary Care. The Influence of Antidepressant Concerns and Treatment Preferences. Prim Care Companion J Clin Psychiatry 2007; 9(2): 91-99.
- Andrews PW, et al: Blue Again. Perturbational Effects of Antidepressants Suggest Monoaminergic Homeostasis in Major Depression. Front Psychol 2011; 2.
- Nierenberg AA, et al: Placebo-controlled continuation treatment with mirtazapine. Acute pattern of response predicts relapse. Neuropsychopharmacology 2004; 29(5): 1012-1018.
- Stewart JW, et al: Use of pattern analysis to predict differential relapse of remitted patients with major depression during 1 year of treatment with fluoxetine or placebo. Arch Gen Psychiatry 1998; 55(4): 334-343.
- DGPPN, et al: for the Unipolar Depression Guideline Group. S3 guideline/national health care guideline unipolar depression – long version, 2nd edition. 2015.
- Spijker J, et al: Duration of major depressive episodes in the general population. Results from The Netherlands Mental Health Survey and Incidence Study (NEMESIS). Br J Psychiatry J Ment Sci 2002; 181: 208-213.
- Glue P, et al: Meta-analysis of relapse prevention antidepressant trials in depressive disorders. Aust N Z J Psychiatry 2010; 44(8): 697-705.
- Kaymaz N, et al: Evidence that patients with single versus recurrent depressive episodes are differentially sensitive to treatment discontinuation. A meta-analysis of placebo-controlled randomized trials. J Clin Psychiatry 2008; 69(9): 1423-1436.
- Viguera AC, et al: Discontinuing antidepressant treatment in major depression. Harv Rev Psychiatry 1998; 5(6): 293-306.
- Farb NAS, et al: Mood-Linked Responses in Medial Prefrontal Cortex Predict Relapse in Patients with Recurrent Unipolar Depression. Biol Psychiatry 2011; 70(4): 366-372.
- Lythe KE, et al: Self-blame-selective hyperconnectivity between anterior temporal and subgenual cortices and prediction of recurrent depressive episodes. JAMA Psychiatry 2015; 72(11): 1119-1126.
- Ferenczi EA, et al: Prefrontal cortical regulation of brainwide circuit dynamics and reward-related behavior. Science 2016; 351(6268): aac9698.
- Huys QJM, et al: Computational psychiatry as a bridge from neuroscience to clinical applications. Nature Neurosci [accepted, publication ahead].
- Huys QJM, et al: Interplay of approximate planning strategies. Proc Natl Acad Sci USA 2015; 112(10): 3098-3103.
InFo NEUROLOGY & PSYCHIATRY 2016; 14(2): 16-19.