Venous thromboembolism (VTE) is the third most common cardiovascular disease in Central Europe. They manifest as deep vein thrombosis in about two-thirds of cases and with symptoms of pulmonary embolism in about one-third. During reproductive age, women are more likely to experience VTE events than men of the same age.
Venous thromboembolism (VTE) is the third most common cardiovascular disease in Central Europe. They manifest as deep vein thrombosis in about two-thirds of cases and with symptoms of pulmonary embolism in about one-third. During reproductive age, women are more likely to experience VTE events than men of the same age. This is mainly attributed to women-specific risk factors such as the use of hormonal contraceptives and pregnancies. Despite this relative increase in risk, the overall risk of VTE remains low, at approximately 2-5 per 10 000 women per year [1].
Hormones and hemostasis
Estrogens affect hepatic gene expression and shift the balance between coagulatory and anticoagulatory factors toward increased coagulability. The level of estrogen dose and the type of progestin in combination preparations determine the risk of thrombotic complications. Increased activities of thrombogenic factors (fibrinogen, prothrombin, factor VII, factor VIII, factor X) and reduced activities of physiological coagulation inhibitors (antithrombin, protein S, tissue factor pathway inhibitor [TFPI]) can be detected when hormone preparations are taken [2,3]. This results in resistance to activated protein C (so-called acquired APC resistance). The extent of this APC resistance correlates with the risk of VTE [4].
Combined hormonal contraceptives
Combined hormonal contraceptives (CHCs) account for the largest proportion of contraceptive methods used. Just a few years after its launch in 1960, CHDs were known to increase the risk of venous thrombosis and, to a lesser extent, arterial thrombosis [5]. The composition of CHD has changed over the past 60 years, but the basic components remain unchanged. Modern CHDs contain a combination of an estrogen (usually ethinyl estradiol at a dosage of 20 -35 µg) and a synthetically produced progestin. The composition determines the extent of the risk increase for thromboembolic events (Table 1) . Among CHDs, preparations containing a low dose of estrogen and levonorgestrel as a progestogen have the lowest risk of VTE [6,7]. With other progestin components, the risk of VTE is significantly higher in some cases. An increased risk of thrombosis has been described not only for the orally applicable CHDs but also for the transdermally and transvaginally applied combination preparations [8].

The risk of thromboembolism is highest in the first months of use and decreases significantly during the first year. However, even with long-term use, an approximately 2-fold increased risk of VTE remains for women using CHD compared with women not using hormonal contraception [9]. If hormonal contraception is paused for several weeks, the risk of VTE is again temporarily higher with resumption than with continuous use. Therefore, it does not seem reasonable to pause CHD perioperatively to reduce the risk of VTE. Therefore, if the risk of VTE is high, VTE prophylaxis with a low-molecular-weight heparin or another substance approved for the indication should rather be given [1].
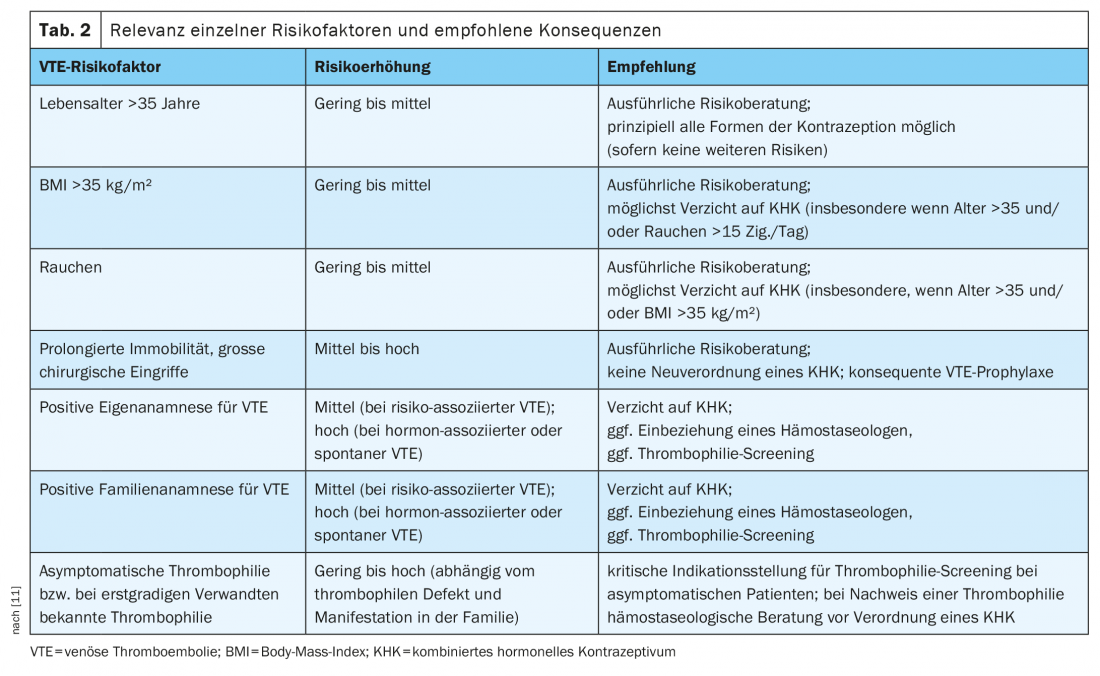
In the presence of additional risk factors (eg, hereditary thrombophilia, positive family history, older age, obesity, smoking), the risk of VTE increases further [10]. Individual risk of VTE should be evaluated each time a CHD is newly prescribed by careful history taking. The recently updated AWMF S3 guideline on hormonal contraception recommends that factors such as age, body mass index, smoking status, mobility limitation, pending major surgery, information on self and family history for VTE events, and known thrombophilia, if any, be included in the risk assessment [11]. An overview of risk factors and recommended consequences is given in Table 2.
Hereditary thrombophilia and hormonal contraception
Whether a positive family history alone is a contraindication to hormonal contraception is controversial. Some studies have shown that hereditary thrombophilia together with the use of a CHD significantly increases the relative risk of VTE [12,13]. The consensus today is that general thrombophilia screening before initial prescription of a CHD is not useful. At least one hereditary thrombophilia can be detected in 3-9% of the central European population (Table 3) . Most frequent are heterozygosity for a factor V Leiden mutation (approx. 2-7%) or a prothrombin G20210A mutation (approx. 1-2%). Despite the high prevalence of these mutations, the absolute risk of VTE in affected individuals is low unless other risk factors are added.

A recent French paper that included 2214 relatives from 651 families with known hereditary thrombophilia and VTE manifestation calculated an annual absolute risk of VTE of 0.36% (HR 1.91; 95% CI 1.30-2.80) for individuals with mild thrombophilia but no previous VTE events of their own and 0.64% (HR 3.78; 95% CI 2.50-5.73) for individuals with severe thrombophilia [14]. However, the risk of VTE increases significantly when a CHD is taken.
Another study group calculated up to a 45-fold increased relative risk of VTE for the use of CHD in women with a factor V Leiden mutation [15]. Women with thrombophilia and a positive family history should therefore not be prescribed CHD if possible, especially if the index patient has had a VTE event at a young age without other risk factors or hormone-associated. If the prescription of a CHD is unavoidable because of concomitant circumstances or comorbidities, hemostaseologic evaluation and prescription of a CHD with levonorgestrel as the progestin component should be considered. CHD should be avoided in cases of known severe thrombophilia; in these cases, preference should be given to an estrogen-free contraceptive method.
Progestin monopreparations
According to current knowledge, contraceptives with a progestogen-only component (i.e., oral preparations containing desogestrel or levonorgestrel or intrauterine devices containing levonorgestrel) do not significantly increase the risk of VTE. Therefore, they may be used in women at increased risk of VTE or with a history of VTE [1,16]. However, this does not apply to depot medroxyprogesterone acetate (DMPA; so-called 3-month injection), for which an approximately 3-fold increased risk of VTE has been described [17]. Therefore, administration of DMPA should be avoided in women at increased risk of VTE.
Contraception under anticoagulation
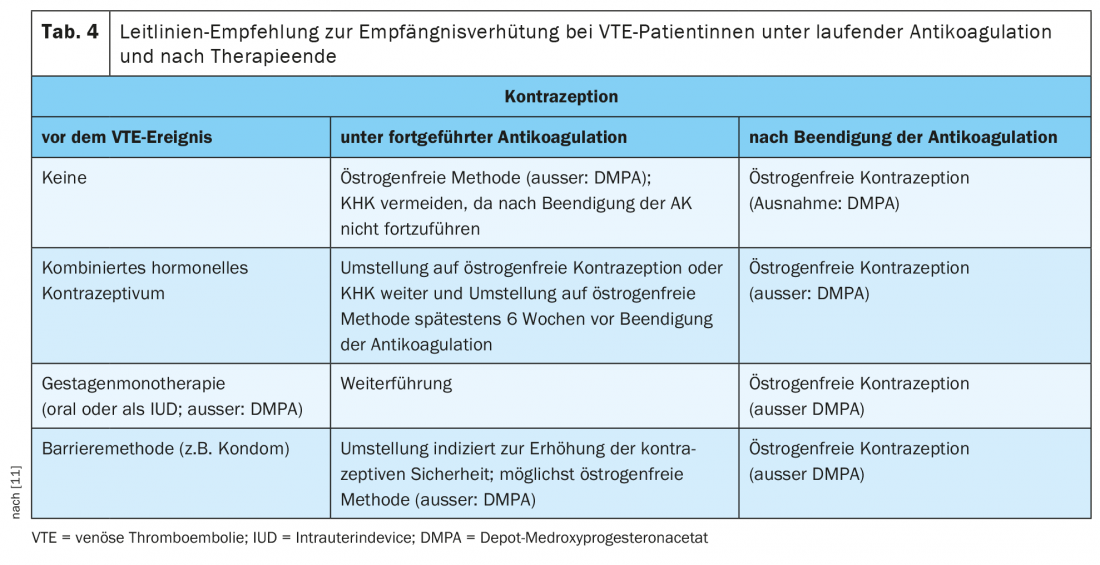
Often, women with hormone-associated VTE stop taking the contraceptive immediately after the diagnosis is confirmed. This is -problematic in that discontinuation leads to cessation bleeding, which can be more severe with higher-dose anticoagulants in the initial phase (apixaban, rivaroxaban) or overlapping anticoagulation (NMH plus vitamin K antagonist [VKA]) than in the maintenance therapy phase. In addition, the risk of unwanted pregnancy increases. The current AWMF S3 guideline calls for safe contraception for all women on oral anticoagulant therapy [11], as both direct oral anticoagulants (DOAKs) and VKA are placenta permeable and thus potentially embryotoxic. The current assessment is that the prothrombogenic effect of CHD is compensated by fully therapeutic anticoagulation, so continued contraception with a CHD under anticoagulation protection is considered safe.
In a post hoc subgroup analysis of the EINSTEIN-DVT and EINSTEIN-PE trials comparing the risk of recurrence in women before 60 years of age with and without continued hormone therapy, there was no evidence of an increased rate of VTE recurrence with continued hormone therapy (3.7% vs. 4.7%; HR 0.56; 95% CI 0.23-1.39) [18]. To minimize the risk of VTE during anticoagulation, the AWMF S3 guideline recommends a progestin monopreparation (oral or intrauterine device) or a copper IUD as the first choice (estrogen-free methods). (Tab. 4). If the patient, together with her physician, decides to continue contraception with a CHD, a switch to a preparation with levonorgestrel as the progestogen component is recommended [11].
Hormone-associated VTE and risk of recurrence.
According to current risk assessments, CHDs are considered weak, transient risk factors [19]. Because continued use of CHD after cessation of anticoagulation is accompanied by a presumed high risk of recurrence, CHD should be discontinued or converted to estrogen-free contraception at least 6 weeks before planned cessation of anticoagulation.
In general, women have a lower risk of recurrence after an initial VTE event than men of the same age. Within one year, 5.3% and within 5 years, 11.1% of all women experience a recurrence. In this regard, the risk of recurrence is lower after hormone-associated VTE than after spontaneous VTE (HR 0.5; 95% CI 0.3-0.8) [20]. Cohort studies report an annual absolute risk of 1.1-2.5% [21–23]. This compares with an average risk of bleeding under fully therapeutic anticoagulation of about 1-3% per year [24]. Some studies even report a higher risk of severe and clinically relevant bleeding in women compared to men [10].
Therefore, in consideration of the benefits and risks, anticoagulation after CHD-associated VTE and in the absence of persistent risk factors is usually limited to 3-6 months. If contraception was continued with a CHD, care should be taken to switch it to an estrogen-free contraceptive method at least 6 weeks before planned discontinuation of anticoagulation.
Assisted reproductive techniques and VTE risk.
The proportion of pregnancies resulting from assisted reproductive technologies (ART) has increased in recent years. The hormone therapy required for this increases the risk of VTE. Thus, women in whom pregnancy occurred after in vitro fertilization (IVF) have about a 2-fold higher risk than women with spontaneous onset of pregnancy [25,26]. In a population-based Swedish analysis of 140 458 records from 1990 to 2008, the absolute risk of VTE was 0.42% for pregnant women after IVF treatment compared with 0.25% for women with spontaneous onset of pregnancy (HR 1.77; 95% CI 1.41-2.23). The risk of VTE remained higher throughout the course of pregnancy but was highest in the 1st trimester (HR 4.22; 95% CI 2.46 -7.26) [25]. It is particularly high (1- 4%) for women who develop severe ovarian hyperstimulation syndrome (OHSS) during the course [27,28].
Hormone replacement therapy and VTE risk
Hormone replacement therapy (HRT) is used to treat menopausal symptoms and diseases caused by estrogen deficiency. HRT does not aim to maintain previous hormone levels, but aims to eliminate symptoms such as hot flashes, sleep disturbances, depressive mood or urogenital symptoms due to mucosal atrophy with the lowest effective dose. Since estrogen monotherapy is associated with an increased risk of endometrial cancer, combination preparations are usually used.
Both estrogen monopreparations and estrogen-progestin combination preparations are associated with an approximately 2-3-fold increased risk of VTE [29]. For women with previous VTE, even an approximately 4-fold increase in risk has been described [30,31]. As with hormonal contraception, the risk of VTE increases with the dose of estrogen and depending on the progestogen component (Fig. 1). The risk is highest in the first year of use and remains elevated for the duration of use. It increases with older age and obesity. Unlike for CHD, the risk does not appear to be increased with transdermal application [32,33].

Conclusion for practice
Knowing the increased risk for thromboembolic events, a composition with the lowest known risk of thrombosis should be preferred when prescribing a CHD. In addition, any woman using a CHD should be informed of the increased risk of thromboembolism. The same applies to women who are using artificial insemination methods or who are scheduled for HRT during the menopause. Before prescribing, additional individual risk factors should be investigated, such as venous and arterial thrombosis events in the patient’s own or family history, a known thrombophilia, smoking, or obesity. The woman should also be informed about which signs indicate thrombosis or pulmonary embolism, so that she can seek medical clarification immediately if she experiences the corresponding symptoms.
Take-Home Messages
- Therapy with combination preparations of estrogen and progestin increases the risk of thromboembolism, whereby the extent of the risk increase depends on the estrogen dose as well as the progestin component.
- The risk of VTE is highest in the first year of use but remains elevated thereafter compared with women not taking CHD.
- Progestogen monopreparations (oral or IUD) do not increase the risk of thrombosis according to current knowledge.
- Current guidelines recommend safe contraception in cases of proven VTE; continued CHD use is possible as long as the patient is on fully therapeutic anticoagulation.
- After a hormone-associated VTE event, the risk of recurrence is low provided that therapy with a combination drug was discontinued before anticoagulation was stopped.
Literature:
- Rott H: Birth Control Pills and Thrombotic Risks: Differences of Contraception Methods with and without Estrogen. Hamostaseology 2019; 39(1): 42-48; doi: 10.1055/s-0039-1677806.
- Tchaikovski SN, Rosing J: Mechanisms of estrogen-induced venous thromboembolism. Thrombosis Research 2010; 126(1): 5-11; doi: 10.1016/j.thromres.2010.01.045.
- Oger E, Alhenc-Gelas M, Lacut K, et al: Differential effects of oral and transdermal estrogen/progesterone regimens on sensitivity to activated protein C among postmenopausal women: a randomized trial. Arterioscler Thromb Vasc Biol 2003; 23(9): 1671-1676; doi: 10.1161/01.ATV.0000087141.05044.1F.
- Tans G, van Hylckama Vlieg A, Thomassen MCLGD, et al: Activated protein C resistance determined with a thrombin generation-based test predicts for venous thrombosis in men and women. Br J Haematol 2003; 122(3): 465-470; doi: 10.1046/j.1365-2141.2003.04443.x.
- Tyler ET: Oral contraception and venous thrombosis. JAMA 1963; 185(2): 131; doi: 10.1001/jama.1963.03060020091034.
- Dragoman MV, Tepper NK, Fu R, et al: A systematic review and meta-analysis of venous thrombosis risk among users of combined oral contraception. Int J Gynaecol Obstet 2018; 141(3): 287-294; doi: 10.1002/ijgo.12455.
- Bastos M de, Stegeman BH, Rosendaal FR, et al: Combined oral contraceptives: venous thrombosis. Cochrane Database Syst Rev 2014; (3): CD010813; doi: 10.1002/14651858.CD010813.pub2.
- Lidegaard O, Nielsen LH, Skovlund CW, et al: Venous thrombosis in users of non-oral hormonal contraception: follow-up study, Denmark 2001-10. BMJ 2012; 344: e2990; doi: 10.1136/bmj.e2990.
- Lidegaard Ø, Nielsen LH, Skovlund CW, et al: Risk of venous thromboembolism from use of oral contraceptives containing different progestogens and oestrogen doses: Danish cohort study, 2001-9. BMJ 2011; 343: d6423; doi: 10.1136/bmj.d6423.
- Bistervels IM, Scheres LJJ, Hamulyák EN, et al: Sex matters: practice 5P’s when treating young women with venous thromboembolism. J Thromb Haemost 2019; 17(9): 1417-1429; doi: 10.1111/jth.14549.
- AWMF S3 Guideline. Hormonal contraception. AWMF register number 015/015.
- van Vlijmen EFW, Wiewel-Verschueren S, Monster TBM, et al: Combined oral contraceptives, thrombophilia and the risk of venous thromboembolism: a systematic review and meta-analysis. J Thromb Haemost 2016; 14(7): 1393-1403; doi: 10.1111/jth.13349.
- Wu O, Robertson L, Langhorne P, et al: Oral contraceptives, hormone replacement therapy, thrombophilias and risk of venous thromboembolism: a systematic review. The Thrombosis: Risk and Economic Assessment of Thrombophilia Screening (TREATS) Study. Thromb Haemost 2005; 94(1): 17-25; doi: 10.1160/TH04-11-0759.
- Suchon P, Resseguier N, Ibrahim M, et al: Common Risk Factors Add to Inherited Thrombophilia to Predict Venous Thromboembolism Risk in Families. TH Open 2019; 3(1): e28-e35; doi: 10.1055/s-0039-1677807.
- Hugon-Rodin J, Horellou MH, Conard, J et al: Type of Combined Contraceptives, Factor V Leiden Mutation and Risk of Venous Thromboembolism. Thromb Haemost 2018; 118(5): 922-928; doi: 10.1055/s-0038-1641152.
- Klok FA, Barco S: Optimal management of hormonal contraceptives after an episode of venous thromboembolism. Thrombosis Research 2019; 181: S1-S5; doi: 10.1016/S0049-3848(19)30357-3.
- van Hylckama Vlieg A, Helmerhorst FM, Rosendaal FR: The risk of deep venous thrombosis associated with injectable depot-medroxyprogesterone acetate contraceptives or a levonorgestrel intrauterine device. Arterioscler Thromb Vasc Biol 2010; 30(11): 2297-2300; doi: 10.1161/ATVBAHA.110.211482.
- Martinelli I, Lensing AWA, Middeldorp S, et al: Recurrent venous thromboembolism and abnormal uterine bleeding with anticoagulant and hormone therapy use. Blood 2016; 127(11): 1417-1425; doi: 10.1182/blood-2015-08-665927.
- Kearon C, Ageno W, Cannegieter SC, et al: Categorization of patients as having provoked or unprovoked venous thromboembolism: guidance from the SSC of ISTH. J Thromb Haemost 2016; 14(7): 1480-1483; doi: 10.1111/jth.13336.
- Douketis J, Tosetto A, Marcucci M, et al. Risk of recurrence after venous thromboembolism in men and women: patient-level meta-analysis. BMJ 2011; 342: d813; doi: 10.1136/bmj.d813.
- Christiansen SC, Lijfering WM, Helmerhorst FM, et al: Sex difference in risk of recurrent venous thrombosis and the risk profile for a second event. J Thromb Haemost 2010; 8(10): 2159-2168; doi: 10.1111/j.1538-7836.2010.03994.x.
- Vaillant-Roussel H, Ouchchane L, Dauphin C, et al: Risk factors for recurrence of venous thromboembolism associated with the use of oral contraceptives. Contraception 2011; 84(5): e23-30; doi: 10.1016/j.contraception.2011.06.008.
- Eischer L, Eichinger S, Kyrle PA: The risk of recurrence in women with venous thromboembolism while using estrogens: a prospective cohort study. J Thromb Haemost 2014; 12(5): 635-640; doi: 10.1111/jth.12528.
- van Es N, Coppens M, Schulman S, et al: Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood 2014; 124(12): 1968-1975; doi: 10.1182/blood-2014-04-571232.
- Henriksson P, Westerlund E, Wallén H, et al: Incidence of pulmonary and venous thromboembolism in pregnancies after in vitro fertilisation: cross sectional study. BMJ 2013; 346: e8632; doi: 10.1136/bmj.e8632.
- Rova K, Passmark H, Lindqvist PG: Venous thromboembolism in relation to in vitro fertilization: an approach to determining the incidence and increase in risk in successful cycles. Fertil Steril 2012; 97(1): 95-100; doi: 10.1016/j.fertnstert.2011.10.038.
- Sennström M, Rova K, Hellgren M, et al: Thromboembolism and in vitro fertilization – a systematic review. Acta Obstet Gynecol Scand 2017; 96(9): 1045-1052; doi: 10.1111/aogs.13147.
- Bates SM, Greer IA, Middeldorp S, et al: VTE, thrombophilia, antithrombotic therapy, and pregnancy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(2 Suppl): e691S-e736S; doi: 10.1378/chest.11-2300.
- Canonico M, Plu-Bureau G, Lowe GDO, et al: Hormone replacement therapy and risk of venous thromboembolism in postmenopausal women: systematic review and meta-analysis. BMJ 2008; 336(7655): 1227-1231; doi: 10.1136/bmj.39555.441944.BE.
- Cushman M, Kuller LH, Prentice R, et al: Estrogen plus progestin and risk of venous thrombosis. JAMA 2004; 292(13): 1573-1580; doi: 10.1001/jama.292.13.1573.
- Høibraaten E, Qvigstad E, Arnesen H, et al: Increased risk of recurrent venous thromboembolism during hormone replacement therapy – results of the randomized, double-blind, placebo-controlled estrogen in venous thromboembolism trial (EVTET). Thromb Haemost 2000; 84(6): 961-967.
- Roach REJ, Lijfering WM, Helmerhorst FM, et al: The risk of venous thrombosis in women over 50 years old using oral contraception or postmenopausal hormone therapy. J Thromb Haemost 2013; 11(1): 124-131; doi: 10.1111/jth.12060.
- Renoux C, Dell’Aniello S, Suissa S: Hormone replacement therapy and the risk of venous thromboembolism: a population-based study. J Thromb Haemost 2010; 8(5): 979-986; doi: 10.1111/j.1538-7836.2010.03839.x.
- Mannucci PM, Franchini M: Classic thrombophilic gene variants. Thromb Haemost 2015; 114(5): 885-889; doi: 10.1160/TH15-02-0141.
- Linnemann B, Hart C: Laboratory Diagnostics in Thrombophilia. Hamostaseology 2019; 39(1): 49-61; doi: 10.1055/s-0039-1677840.
- www.bfarm.de/DE/Arzneimittel/Pharmakovigilanz/KOK/_node.html; retrieved on: 7.12.2020.
HAUSARZT PRAXIS 2021; 16(1): 8-13