Oncological therapies are undergoing rapid development, resulting in the availability of a large number of new substances that need to be characterized not only in terms of their potential uses but also in terms of the adverse effects that can be expected. In keeping with the nature of many classical chemotherapies in oncology, it is primarily rapidly dividing tissues that are affected by adverse effects. In recent years, however, it has also been possible to make the immune system therapeutically useful against malignant cells. This results in a new spectrum of side effects, which clearly differs in its development and clinical presentation from classical chemotherapy.
Alopecia
Alopecia is the most common side effect of chemotherapy, occurring in about half of all therapies. In this case, anagen dystrophic efluvium occurs more frequently due to abrupt termination of the hair follicle cycle as a result of the toxic effect on the rapidly dividing keratinocytes in the epithelial matrix of the hair bulb. The severity of alopecia depends on the drug dose, half-life of the drug and duration of the entire therapy. The main causes are alkylants, anthracyclines, tumor antibiotics, and topo- isomerase inhibitors [1]. Because stem cells divide slowly, they are usually unaffected, and alopecia is usually reversible. Exceptions may include high-dose therapies with endoxan and busulfan in combination with total body irradiation in preparation for bone marrow transplantation.
Cooling of the scalp may be considered as a possible preventive intervention [2]. As part of a feasibility study at the Breast Center Zurich, controlled cooling of the scalp with a cooling cap system (Sysmex DigniCap®) was investigated during the administration of cytostatic drugs (Fig. 1).
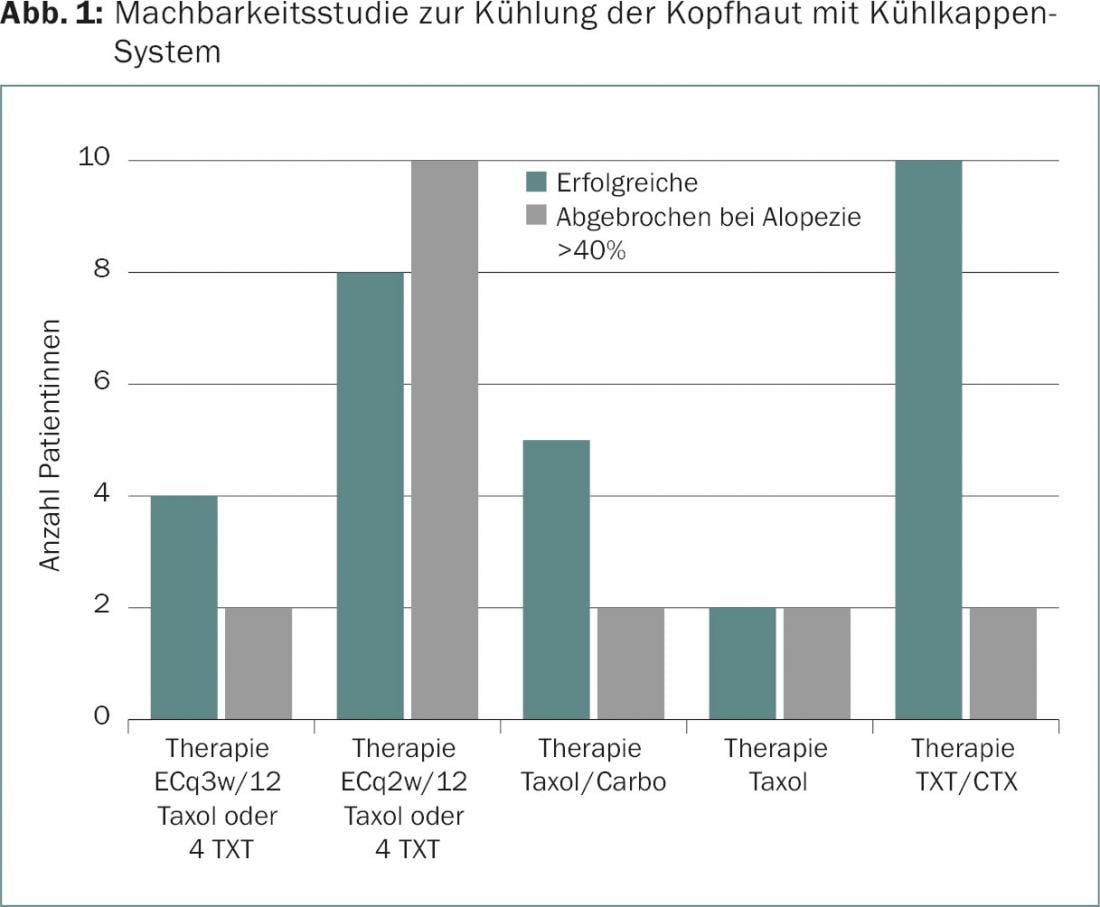
The mode of action is based, on the one hand, on a constriction of the blood vessels in the scalp and the associated reduction in the amount of medication reaching the hair roots. In addition, the absorption of toxic active ingredients into the hair cells is reduced by the low temperatures. In an interim analysis, patients showed hardly any relevant or only transient side effects, such as headache, feeling cold, feeling of heaviness of the head, neck pain, and redness of the skin. According to available studies, the fear that scalp metastases occur more frequently as a result of scalp cooling treatment is not justified, since within the specified observation periods metastases did not occur more frequently than with chemotherapy without such scalp cooling [3]. Depending on the composition of chemotherapy, scalp hair was successfully preserved in a cosmetically pleasing manner in more than half of the patients.
Skin and mucous membrane toxicity
Cytostatic drugs: Especially antimetabolites (5-FU, MTX, Pemetrexed) and tumor antibiotics (DOX, IDA, EPR, BLE) but also taxanes cause stomatitis in a dose-dependent manner. It usually heals spontaneously, although aplasia can lead to complicating infections. Additional risk factors include periodontal disease, caries, poor nutritional status, and xerostomia secondary to radiotherapy. Other mucosal toxic agents may cause cystitis (cyclophosfamide, ifosfamide, rarely etopophos) or enteritis (5-FU, capecitabine, irinotecan, methotrexate, raltitrexet, rarely epiphyllotoxin, cytosine arabinoside, and cisplatin). Substances such as the antifolate pemetrexed (Alimta®), used in monotherapy or combination therapy, produce mucocutaneous side effects (exanthema 30%, mucositis and diarrhea 5%) [4]. Although cutaneous side effects are often reported nonspecifically as “skin-rash,” specific findings and diagnoses can be observed. Thus, alopecia, urticarial vasculitis, acute generalized exanthematous pustulosis, toxic epidermal necrolysis, radiation recall dermatitis, and pityriasis lichenoides occur. The skin changes usually occur shortly after the start of therapy [5].
EGFR inhibitors: EGFR inhibitors such as erlotinib, gefitinib, cetuximab or panitumumab have been successfully used for a number of solid tumors for several years. Characteristic papulo-pustular exanthema, paronychia, xeroderma, pruritus, and alopecia occur in 50-100% of patients [6,7]. Also, the combination of lapatinib (Tyverb®; pan-HER TKI) and capecitabine (Antifolate) regularly leads to mild and sometimes severe changes in skin and mucous membranes. This includes rash (47%), pruritus, paronychia, and stomatitis, but also pustules, hand-foot syndrome (54%), blistering, and diarrhea (grades 3-4, up to 20%) and interstitial pneumonitis, and requires proactive management as well as dermatologic consultation with clarification regarding drug allergy [8].
CDK4/6 inhibitors: CDK4/6 (cyclin-dependent kinase) inhibitors, which are used in combination with anti-hormones such as aromatase inhibitors in breast cancer and are also used in a subtype of liposarcoma, represent another promising substance class [9]. From the first generation of these inhibitors, flavopiridol is the best studied compound. Toxicities correspond to those of classical cytostatic drugs with inflammation at the infusion site, gastrointestinal side effects and severe neutropenia.
For example, a newer inhibitor under clinical investigation is palbociclib, which has had FDA approval since February 2015 as first-line therapy in advanced, postmenopausal ER+/Her2-negative breast cancer in combination with letrozole. Side effects were mild to moderate, with myelosuppression more often dose-limiting.
m-TOR inhibitor: Also in breast cancer patients, for example, the use of the mTOR inhibitor everolimus together with letrozole (aromatase inhibitor) was investigated in the Bolero 2 study [10]. By forming a complex with the protein mTOR (“mammalian target of rapamycin”), this is inactivated. mTOR is part of the two protein complexes mTORC1 and mTORC2, which drive, among other things, the transcription and translation of various proteins and thus the proliferation of tumor cells, but also of T lymphocytes, via various signal transduction pathways. Inhibition of mTOR thus prevents both activation and progression of T cells from G1 phase to S phase of the cell cycle. Progression-free survival of patients as the primary endpoint showed a clear advantage for the combination of both agents over letrozole monotherapy. However, a spectrum of serious side effects emerged. In the BOLERO study, stomatitis (56%) diarrhea (30%) rash (36%) and non-infectious pneumonitis (12%) frequently required treatment and sometimes led to discontinuation of therapy. Patients must therefore pay attention to oral hygiene and prophylactically use a mouth rinse and report respiratory problems immediately.
Immunotherapy – checkpoint inhibitors: With prolonged overall survival in metastatic melanoma due to treatment with the immune checkpoint inhibitor ipilimumab (anti-CTLA4, Yervoy®), the starting signal for the substance class of checkpoint inhibitors, i.e. antibodies that block the checkpoints CTLA-4, PD-1 and PD-1L, was given in 2010 (Fig. 2).
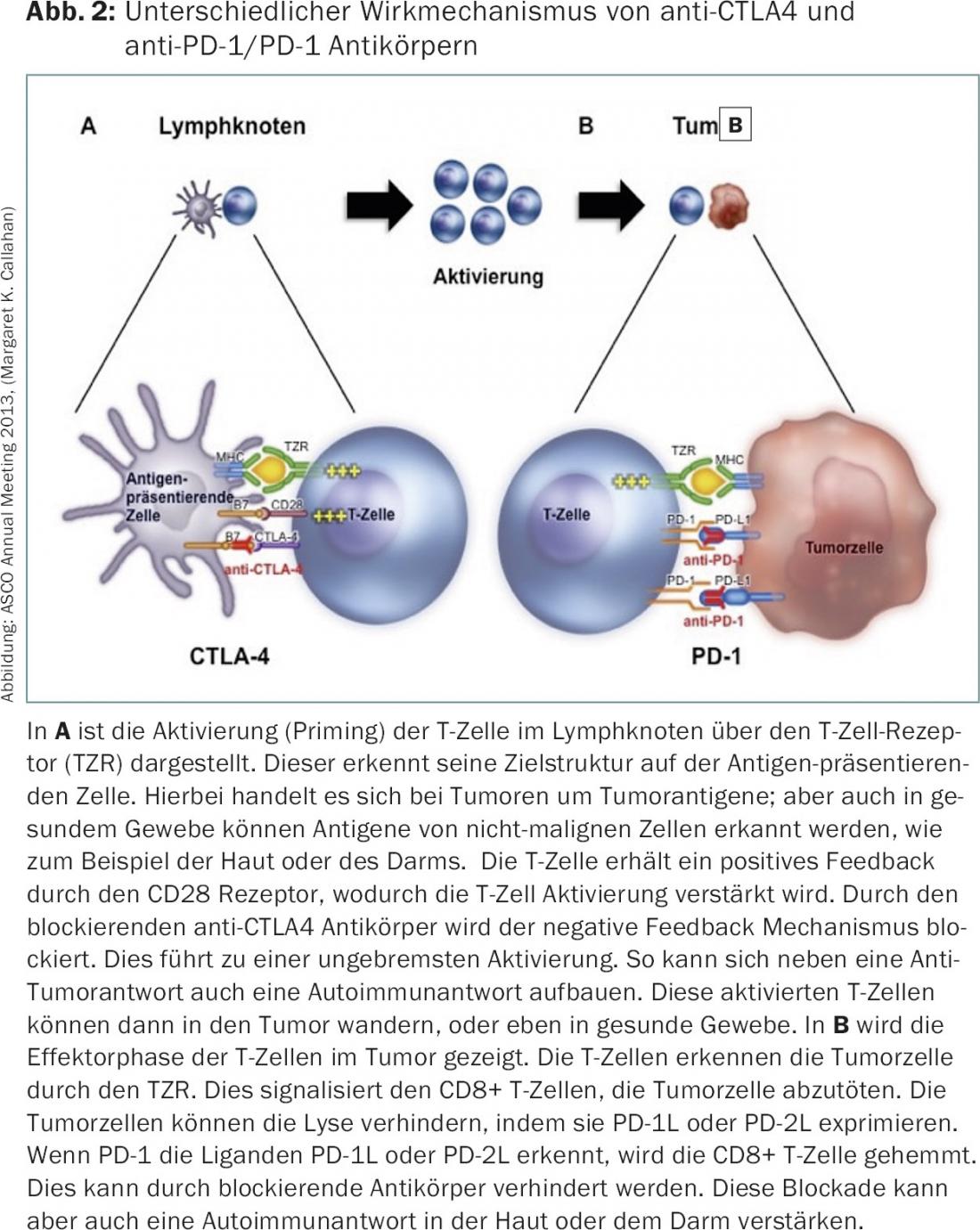
Checkpoint inhibitors have been used so far in various tumors such as bronchial carcinomas [11], renal cell carcinomas [12], melanomas [13], head and neck tumors [14] and bladder carcinomas [15]. However, the initial development was strongly influenced by the new side effects of the checkpoint inhibitors and almost prevented their success story. About 5-15% of patients experienced severe side effects, which can be explained by activation of the immune system against healthy tissues. Thus, iatrogenically induced autoimmunity develops, with the skin and gastrointestinal tract being particularly affected. Both organ systems are also characterized under physiological conditions by the fact that there is an intensive interplay between the immune system and the organ system. In order not to let autoimmunity develop, the checkpoints are of great importance under physiological conditions. So it is not surprising that the blockade of the checkpoints leads to side effects here in particular.
Immunologic changes of the skin in patients on checkpoint inhibitors [16] include pruritus in up to 30%, morbilliform exanthema (10-50%), and vitiligo-like depigmentation. Milder exanthemas can be treated with local skin care measures and local steroids. However, if large maculo-papular exanthemas and possibly blistering occur, especially on the mucous membranes, checkpoint inhibitors must be stopped immediately and systemic therapy with steroids started. In case of severe muco-cutaneous side effects, checkpoint inhibitors must not be reintroduced. Dose reduction is not a measure to improve tolerability because there is no clear dose-response relationship in the substance class of checkpoint inhibitors. This is particularly true in the case of an immune system already activated by checkpoint inhibitors. For this reason, in the event of severe side effects, the checkpoint inhibitor must be paused until the side effects have resolved. After that, a restart must be carefully considered. Less common skin lesions include prurigo nodularis, lichenoid exanthema, papulo-pustular eruptions, ulcers, photosensitivity, and radiation recall [17].
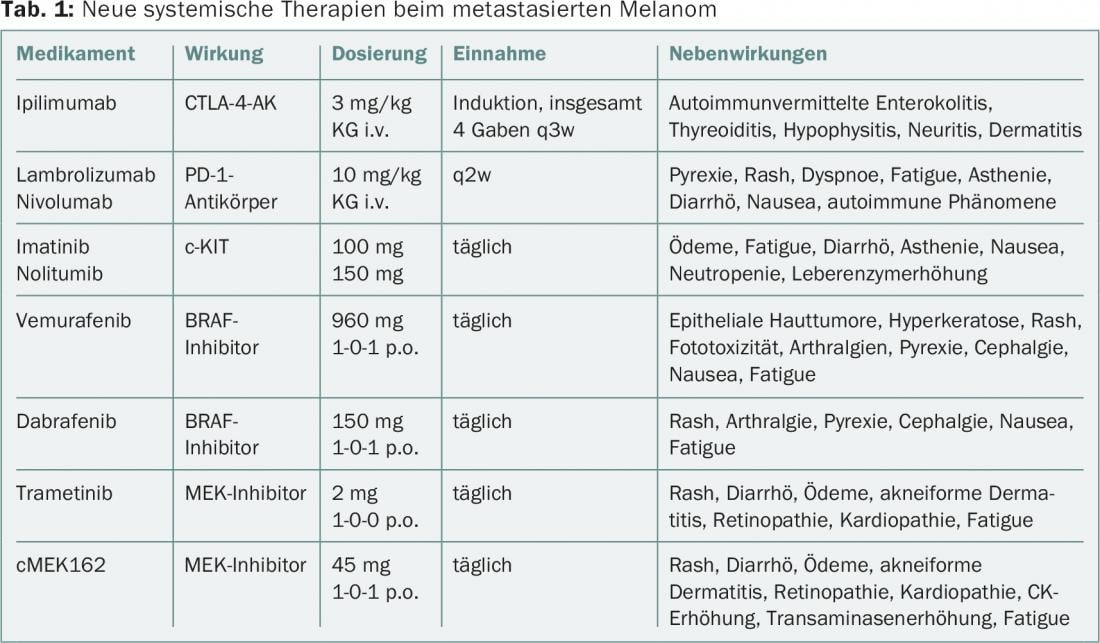
In melanoma, the use of so-called “targeted” therapeutics has advanced rapidly, so that in addition to checkpoint inhibitors, combination therapies are now also being tested [18]. An overview of systemic therapies, effects, and side effects, including those on skin and mucous membranes, is provided in Table 1. The cutaneous side effects may include inflammatory dermatoses, keratoses, and benign and malignant squamous neoplasia in addition to the so-called rash.
In conclusion, when in doubt, if mucocutaneous toxicity from checkpoint inhibitors occurs, the next dose should be skipped. The clinical course then determines which measures must be initiated. If anything is unclear, the treating oncologist should be contacted quickly. This is where timely recording of symptoms using electronic and patient-related systems (APPs) could be useful in the future [19].
Literature:
- Chon SY, et al: Chemotherapy-induced alopecia. J Am Acad Dermatol. 2012; 67: e37-47.
- Young A, et al: The use of scalp cooling for chemotherapy-induced hair loss. Br J Nurs. 2016; 25: S22.
- van den Hurk CJ, et al: Scalp cooling for hair preservation and associated characteristics in 1411 chemotherapy patients – results of the Dutch Scalp Cooling Registry. Acta Oncol. 2012; 51(4): 497-504.
- Trojan A, et al: Adverse chemotherapy effects on skin and mucous membranes. Review. Praxis (Bern 1994). 2002; 91(24): 1078-87.
- Piérard-Franchimont C, et al: Revisiting cutaneous adverse reactions to pemetrexed. Oncol Lett. 2011; 2: 769-772.
- Gerber PA, et al: Therapy with epidermal growth factor receptor inhibitors. Spectrum of cutaneous side effects Dermatologist. 2010; 61: 654-61.
- Macdonald JB, et al: Cutaneous adverse effects of targeted therapies: Part I: Inhibitors of the cellular membrane. J Am Acad Dermatol. 2015; 72: 203-18.
- Bachelot T, et al: Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol. 2013; 14(1): 64-71.
- Finn RS, et al: Targeting the cyclin-dependent kinases (CDK) 4/6 in estrogen receptor-positive breast cancers. Breast Cancer Res. 2016; 18: 17.
- Piccart M, et al: Everolimus plus exemestane for hormone-receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: overall survival results from BOLERO-2. Ann Oncol. 2014; 25(12): 2357-62.
- Herzberg B, et al: Immune checkpoint inhibitors in non-small cell lung cancer. Oncologist. 2016.
- Carlo MI, et al: Checkpoint inhibitors and other novel immunotherapies for advanced renal cell carcinoma. Nat Rev Urol. 2016; 13(7): 420-31.
- Redman JM, et al: Advances in immunotherapy for melanoma. BMC Med. 2016; 14: 20.
- Fuereder T.: Immunotherapy for head and neck squamous cell carcinoma. Memo. 2016; 9: 66-69.
- Zhou TC, et al: Review of the PD-1/PD-L1 checkpoint in bladder cancer: From mediator of immune escape to target for treatment. Urol Oncol. 2016
- de Golian E, et al: Cutaneous Complications of Targeted Melanoma Therapy. Curr Treat Options Oncol. 2016; 17: 57.
- Macdonald JB, et al: Cutaneous adverse effects of targeted therapies: Part II: Inhibitors of intracellular molecular signaling pathways. J Am Acad Dermatol. 2015; 72: 221-36.
- Trojan A, et al: Progress in metastatic malignant melanoma. Dermatology Practice 2014; Vol. 24, No. 3.
- Egbring M, et al: A Mobile App to Stabilize Daily Functional Activity of Breast Cancer Patients in Collaboration With the Physician: A Randomized Controlled Clinical Trial. J Med Internet Res. 2016; 18(9).
InFo ONCOLOGY & HEMATOLOGY 2016; 4(7-8): 19-22.