In a symposium at the 26th Physicians’ Continuing Education Course in Clinical Oncology in St. Gallen, various forms of radiotherapy were explained. In recent years, technological progress has led to the rapid development of radiation treatment. Patients benefit from this: The therapies are often less burdensome (fewer radiation appointments), trigger side effects less frequently, and for various indications, radiotherapy can significantly improve local tumor control and also overall survival.
Prof. Matthias Guckenberger, MD, University Hospital Zurich, presented the possibilities of stereotactic radiotherapy (SRT). In this procedure, a relatively small volume that is clearly delineated against adjacent tissue is irradiated in high doses and from multiple directions. In this way, the surrounding tissue is optimally protected. SRT is best established for the treatment of brain tumors and metastases – in a limited number, additional whole-brain irradiation is obsolete because it can impair patients’ cognition.
Possibilities and limitations of stereotactic irradiation
In recent years, SRT has been further developed, and it can now also be used successfully in the trunk of the body as body stereotaxy (SBRT). SBRT is used in both primary and metastatic settings, most notably for primary therapy of stage I non-small cell lung cancer (NSCLC). An analysis of 582 NSCLC patients treated in 13 German radiotherapy centers between 1998 and 2011 showed a local tumor control of more than 80% and an overall survival of 47% after a follow-up of three years [1]. These results are consistent with the results of other studies. Pooled analysis of two randomized trials showed equivalence of SBRT and lobectomy, making SBRT the treatment of choice today in cases of inoperability or patient rejection of surgery [2].
Since the turn of the millennium, the number of centers offering SBRT for NSCLC has increased dramatically, and with it the number of patients treated accordingly. In patients with stage I NSCLC over 75 years of age treated with radiotherapy, OS increased significantly from 2001 to 2007 [3].
The use of SBRT is also being investigated in prostate cancer. However, the optimal radiation dose for this indication has not yet been determined. In a dose-finding study, severe grade 3 and 4 toxicities were seen in 10% of patients at the highest radiation doses (5× 10 Gy), and five of 61 patients underwent colostomy [4]. “These results show that biology cannot be overcome even with technology,” the speaker commented. This is because excellent results in terms of both tumor control and side effect profile have been achieved with SBRT when biologically-adjusted radiation doses are used.
The use of SBRT for lung, liver, and spine metastases is now well established. In the case of single metastases or oligometastasis, local tumor control can be achieved in 80-90% of patients, and in the case of systemic metastasis, at least symptom control can be achieved. Even so-called “radiation-resistant” tumors can be treated and permanently controlled with SBRT if the dose is appropriately high. An interesting further development is the combined administration of local SBRT and systemic targeted therapy. High-dose radiation can apparently stimulate an immune response that, in combination with “targeted therapy,” can have a positive effect on non-irradiated metastases as well.
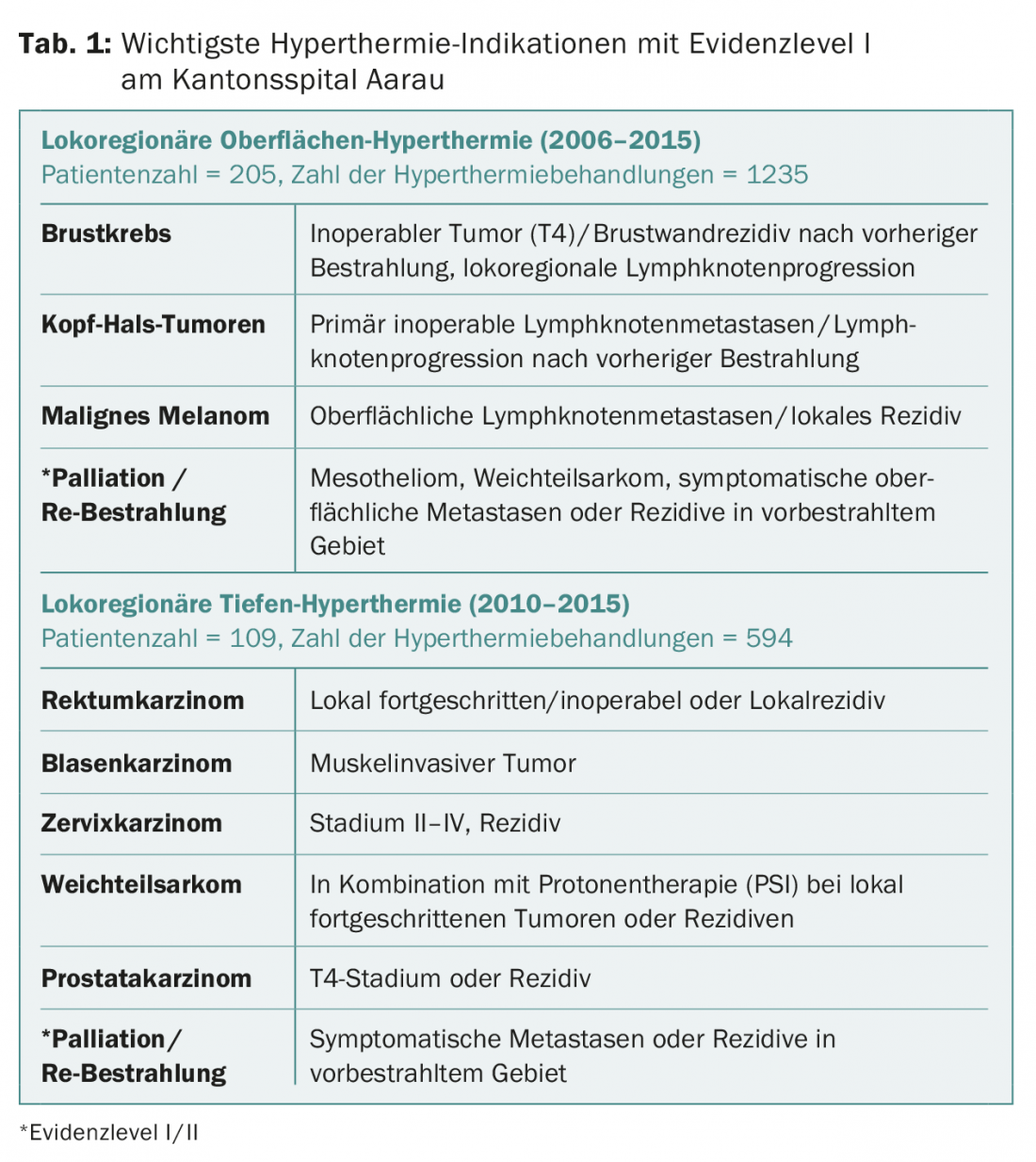
Hyperthermia in radiooncology
Prof. Dr. med. Stephan Bodis, Kantonsspital Aarau, informed about the current indications for hyperthermia treatment. Hyperthermia serves as a “radiosensitizer” before or after radiotherapy. In the process, the area to be irradiated resp. previously irradiated tumor region heated to 39-43 °C. Whether hyperthermia is performed before or after radiation does not matter for the outcome. The qualitative spread of existing studies is enormous. However, there are very good data on individual tumor entities showing that hyperthermia can improve local control by up to 60-80%, for example in bladder cancer or locoregional breast wall recurrences in breast carcinoma.
In Switzerland, hyperthermia applications are performed at the Cantonal Hospital Aarau. As a rule, both hyperthermia and the subsequent radiotherapy are performed in Aarau, in complex situations and a close distance to the referring radiotherapy institute (this must be accessible to the patient within 1.5 hours after hyperthermia) also only the hyperthermia. A surface hyperthermia device is available for the treatment of breast cancer, melanoma, and head and neck tumors, and a deep hyperthermia device is available for the treatment of bladder, rectal, anal, and cervical cancers and sarcomas (Table 1).
Hyperthermia is applied once or twice a week during radiation treatment of breast wall recurrences in breast carcinoma. During 60 minutes, the tissue is heated to 41-43 °C, while vital signs are continuously monitored and temperatures are measured at various points in the region to be treated. Immediately before or after hyperthermia, radiotherapy (20-50 Gy in fractions of 2-4 Gy) is given. The results are encouraging: within the median follow-up of ten months, over 90% of patients had responded to treatment (66.7% complete remission, 25% good partial remission) [5].
Before the start of hyperthermia treatment, the patient is discussed, if possible, at the “Swiss Hyperthermia Tumorboard”, which takes place once a month at the Cantonal Hospital Aarau and in which twelve clinics participate so far. Currently, several studies are ongoing within the “Hyperthermia Research Network Group”, including one on hyperthermia in muscle-invasive bladder carcinoma and the HYPROSAR (“Hyperthermia Protons Sarcomas”) study on the combination of proton therapy and hyperthermia in non-resectable soft tissue sarcomas of adults.
Place of intraoperative radiotherapy
Prof. Dr. med. Felix Sedlmayer, Salzburg (Austria), spoke about a rather rarely used form of radiation treatment, intraoperative radiotherapy (IORT). Defined IORT as the application of a high, single dose of radiation during tumor surgery. The goal of IORT is to improve locoregional tumor control. There are some arguments in favor of IORT (Table 2), but the question is whether it still has a place in today’s world with the greatly improved capabilities of radiation therapy. Clearly, this is the case, as the number of IORT studies has increased in recent years, in part because there is now better access to mobile radiation equipment.

By far the most common indication for IORT (in trials) is breast carcinoma, followed by rectal, gastric, pancreatic, and sarcomas. Corresponding study data are collected in the registry of the International Society of Intraoperative Radiation Therapy (www.isiort.org). Currently, data show that IORT can improve local control in several tumor entities and, in selected indications, can also prolong overall survival. Nevertheless, the evidence regarding IORT is (still) limited. For this reason, it is necessary to conduct more well-designed clinical trials.
Source: 26th Physicians Continuing Education Course in Clinical Oncology, February 18-20, 2016, St. Gallen.
Literature:
- Guckenberger M, et al: Safety and efficacy of stereotactic body radiotherapy for stage 1 non-small-cell lung cancer in routine clinical practice: a patterns-of-care and outcome analysis. J Thorac Oncol 2013; 8(8): 1050-1058.
- Chang JY, et al: Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2015 Jun; 16(6): 630-637.
- Palma D, et al: Impact of introducing stereotactic lung radiotherapy for elderly patients with stage I non-small-cell lung cancer: a population-based time-trend analysis. J Clin Oncol 2010; 28(35): 5153-5159.
- Kim DWN, et al: Predictors of Rectal Tolerance Observed in a Dose-Escalated Phase 1-2 Trial of Stereotactic Body Radiation Therapy for Prostate Cancer. IJROBP 2014; 89: 509-517.
- Datta NR, et al: Hyperthermia and reirradiation for locoregional recurrences in preirradiated breast cancers: a single institutional experience. Swiss Med Wkly 2015; 145: w14133.
InFo ONCOLOGY & HEMATOLOGY 2016; 4(3): 28-30.











