From January 25 – 27, 2024, updated data on first-line maintenance therapy with avelumab (Bavencio®) were presented at the annual American Society of Clinical Oncology Genitourinary Cancers (ASCO GU) Symposium in San Francisco, USA. in locally advanced or metastasized urothelial carcinoma (la/mUC). Results of ongoing real-world studies and patient-reported outcomes (PROs) confirm the efficacy and safety of avelumab while maintaining quality of life [1-4]. The AVENANCE study stood out in particular, which showed a median overall survival (mOS) of 40.8 months from the start of first-line chemotherapy (1L-ChT) in patients treated with antibody-drug conjugates (ADC) after avelumab therapy [1].
In the phase III JAVELIN-Bladder-100 study, first-line treatment with avelumab (Bavencio®) in addition to Best Supportive Care (BSC) provided a survival benefit compared to BSC alone for la/mUC patients who had previously responded to platinum-based 1L-ChT [5]. Since then, the JAVELIN bladder regime has been considered the standard of care in international guidelines [6]. Several real-world studies also confirmed the efficacy and safety of avelumab in a real clinical context [7, 8]. At this year’s ASCO GU Symposium, updated real-world data from ongoing studies and patient reports from JAVELIN-Bladder-100 were presented, which analyzed the efficacy and safety of avelumab even after longer follow-up [1-4]. In addition, the AVENANCE study was the first to analyze overall survival data of patients undergoing second-line (2L) therapy after the JAVELIN bladder regimen [1]. The treatment results of the various 2L therapies in real-world studies provide information on which therapy sequences could achieve the best possible results for patients in a practical clinical setting [1].
Strategic treatment sequences for successful therapy
In his poster, Prof. Philippe Barthélémy from the Institut de Cancérologie Strasbourg in France presented updated data from the ongoing ambispective real-world AVENANCE study, which included la/mUC patients after response to platinum-based 1L-ChT [1]. After a follow-up of 26.3 months (range: 0.6 – 43.7) since the start of avelumab first-line maintenance therapy, the mOS of the 595 patients included was 21.3 months (95% CI: 17.6 – 24.6) from the start of avelumab treatment. At the time of the data cut-off, 125 (21.0%) of the patients were still receiving avelumab treatment, while 330 (55.5%) were receiving 2L therapy. Patients who were treated with an ADC in the 2L after completion of avelumab therapy (56 [17,0%] with enfortumab vedotin and 6 [1,8%] with sacituzumab govitecan) showed an mOS of 31.3 months (95% CI: 29.1 – not assessable [NE]), while patients under ChT in the 2L (81 [24,5%] under platinum-based ChT and 163 [49,4%] under other ChT) had an mOS of 14.4 months (95% CI: 13.2 – 15.9) from the start of avelumab therapy [1].
An exploratory analysis showed an mOS of 26.5 months (95% CI: 23.4 – 30.1) in all included patients calculated from the start of 1L-ChT. For patients treated with ADC in 2L after avelumab first-line maintenance therapy, the mOS from the start of 1L ChT was 40.8 months (95% CI: 32.6 – 42.1) compared to 24.5 months (95% CI: 19.8 – 29.8) with platinum-based ChT in 2L (Fig. 1) [1].
Further real-world studies confirm effectiveness
In addition to Prof. Philippe Barthélémy, Prof. Sergio Bracarda (Azienda Ospedaliera Santa Maria, Terni, Italy) and Dr. Petros Grivas (Fred Hutchinson Cancer Center, Seattle, USA) also presented updated real-world data on first-line maintenance therapy with avelumab [2, 3]. The ongoing READY study includes data from an Italian compassionate use program (CUP) to test the efficacy and safety of avelumab in la/mUC patients (N=464) who were not progressing on platinum-based 1L-ChT [2]. After a median follow-up of 20.3 months (95% CI: 19.8 – 20.9), the mOS calculated from the start of 1L-ChT was 30.9 months (95% CI: 25.7 – NE) [2]. The real-world PATRIOT-II study also included la/mUC patients (N=106) according to the JAVELIN bladder regimen and recorded an mOS of 30.5 months (95% CI: 23.4 – 37.6) after 16.0 months (IQR: 11.0 – 21.0) follow-up from the start of avelumab maintenance therapy calculated from the start of 1L-ChT [3].
Maintained quality of life with avelumab
Dr. Petros Grivas also presented the long-term and exploratory evaluations of PROs from the JAVELIN Bladder 100 study [4]. PROs were assessed at baseline, on day 1 of each 4-week cycle, at the end of treatment or discontinuation of the study, and up to 90 days post-treatment with the NFBlSI-18 and EuroQol EQ-5D-5L. At the time of the data cut-off, the median follow-up was 38.0 months (≥2 years for all patients; N=350), and the median duration of treatment was 5.8 months. PRO levels remained stable during treatment and no clinically significant changes from baseline were observed. Around 75% of the patients who could be evaluated reported no change or a reduction in their symptoms due to side effects [4].
Conclusion
The updated long-term results of the presented real-world studies show similar results to the JAVELIN Bladder 100 study, with an mOS of >30 months from the start of treatment with avelumab (Bavencio®), thus underlining the importance of avelumab as first-line maintenance therapy in la/mUC patients who have previously responded to platinum-based 1L-ChT [2, 3, 9]. According to PROs, patients were able to maintain their quality of life and control of cancer-related symptoms during treatment with avelumab [4]. In addition, the subgroup analysis of the AVENANCE study shows that 2L therapy with ADC after avelumab extends the mOS from the start of 1L ChT to 40.8 months. Thus, the choice of a strategic treatment sequence has the potential to further optimize patient outcomes [1].
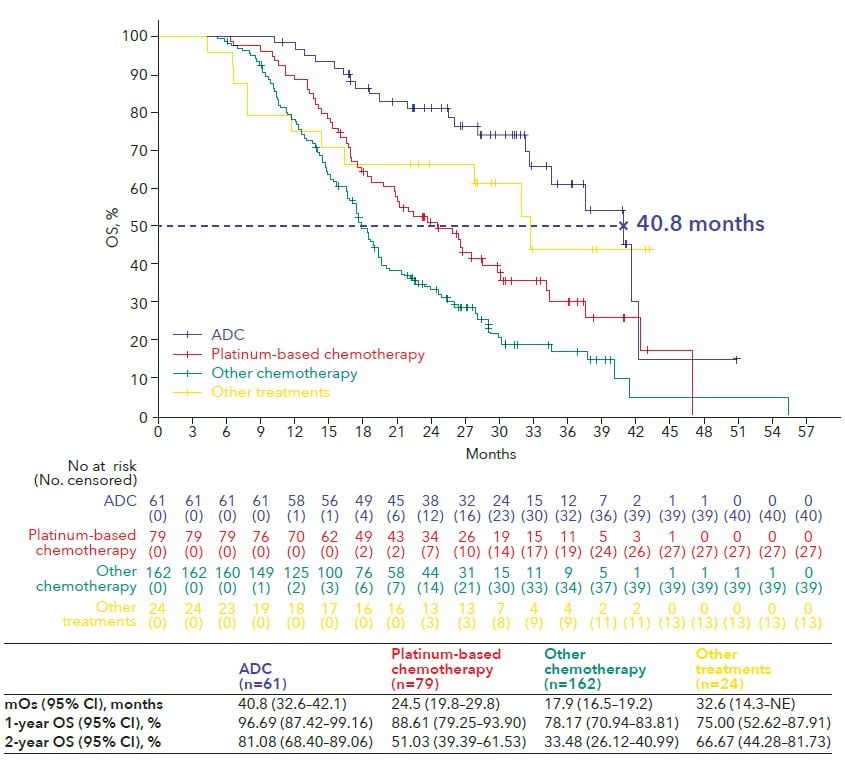
Figure 1) Overall survival (OS) from the start of 1L ChT in the study population without disease progression after 1L ChT, divided into subgroups by 2L treatment (blue = antibody-drug conjugate [ADC]; pink = platinum-based ChT; green = other ChT; orange = other treatments). CI = confidence interval.
Adapted from Barthélémy et al. 2024 [1].
Abbreviations
EuroQol EQ-5D-5L = European Quality of Life 5 Dimensions 5 Level Version; FACT = Functional Assessment of Cancer Therapy; IQR = Interquartile Range; CI = Confidence Interval; NCCN = National Comprehensive Cancer Network; NFBlSI-18-Score = NCCN/FACT Bladder Symptom Index.
The BAVENCIO® brief technical information.
CH-AVE-00084 02/2024
With the financial support of Merck (Schweiz) AG.
Literature
1 Barthélémy P. et al. Updated results from AVENANCE: real-world effectiveness of avelumab first-line maintenance in patients with advanced urothelial carcinoma and analysis of subsequent treatment. Abstract No. 561. Presented at the ASCO Genitourinary Cancers Symposium, January 25-27, 2024; San Francisco, CA.
2 Bracarda S. et al. Subgroup analyses from READY: REAl-world Data from an Italian compassionate use program of avelumab first-line maintenance treatment for locallY advanced or metastatic urothelial carcinoma.Abstract No. 558. Presented at the ASCO Genitourinary Cancers Symposium, January 25-27, 2024; San Francisco, CA.
3 Grivas P. et al. Avelumab first-line maintenance therapy for locally advanced/metastatic urothelial carcinoma: results from the real-world US PATRIOT-II study. Abstract No. 697. Presented at the ASCO Genitourinary Cancers Symposium, January 25-27, 2024; San Francisco, CA.
4 Grivas P. et al. Avelumab first-line maintenance for advanced urothelial carcinoma: long-term patient-reported outcomes in the phase 3 JAVELIN Bladder 100 trial. Abstract No. 581. Presented at the ASCO Genitourinary Cancers Symposium, January 25-27, 2024; San Francisco, CA.
5 Powles, T., et al, Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. NEJM, 2020. 383(13): p. 1218-1230.
6. National Comprehensive Cancer Network® (NCCN Guidelines®). Bladder Cancer (version 3.2023). www.nccn.org/professionals/physician_gls/pdf/bladder.pdf. Last accessed: January 2024.
7 Barthélémy P., et al. Full analysis from AVENANCE: A real-world study of avelumab first-line (1L) maintenance treatment in patients (pts) with advanced urothelial carcinoma (aUC). Abstract No. 471. Presented at the 2023 ASCO Genitourinary Cancers Symposium, February 16-18, 2023; San Francisco, USA.
8 Antonuzzo L., et al. READY: Real-world data from an Italian compassionate use program of avelumab first-line maintenance (1LM) treatment for locally advanced or metastatic urothelial carcinoma (la/mUC). Abstract No. 469. Presented at the 2023 ASCO Genitourinary Cancers Symposium, February 16-18, 2023; San Francisco, USA.
9 Sridhar S., et al. Avelumab first-line (1L) maintenance for advanced urothelial carcinoma (UC): Long-term follow-up from the JAVELIN Bladder 100 trial in subgroups defined by 1L chemotherapy regimen and analysis of overall survival (OS) from start of 1L chemotherapy. Abstract No. 508. Presented at the 2023 ASCO Genitourinary Cancers Symposium, February 16-18, 2023; San Francisco, USA.
The references are available on request.
Article online since 23.02.2024