A patient with type 2 diabetes is 2-3 times more likely to have a myocardial infarction or stroke than people without diabetes. Cardiovascular disease accounts for up to 50% of diabetes-associated deaths. Optimal treatment of the four main risk factors blood glucose, weight, hypertension and blood lipid levels is therefore indicated.
Although coronary heart disease mortality in patients with type 2 diabetes has steadily decreased over the past 2 decades, they are approximately 2- to 3-fold more likely to have a myocardial infarction or stroke than people without diabetes [1]. Cardiovascular disease thus accounts for up to 50% of diabetes-associated deaths [2]. Despite this increased risk, most affected individuals are still not adequately treated with regard to the four main risk factors of blood glucose, weight, hypertension, and blood lipid levels, and only about one in 300 diabetic patients reaches their individual target values [3]. It is this multifactorial therapy of all risk factors that makes the adequate therapy of a patient with type 2 diabetes a daily challenge in practice. The following article is intended to provide an update on current treatment recommendations for this.
Platelet aggregation inhibition
For a long time, the administration of acetylsalicylic acid (ASA) 100 mg was also considered a useful measure in the primary prevention of type 2 diabetes, because the administration was associated with a reduction in ischemic events. In a meta-analysis that included patients with and without diabetes, treatment resulted in a relative reduction of major adverse cardiac events (MACE) by 12% (0.51% vs. 0.57%, p≤0.0001) in individuals in low-risk primary prevention, primarily by reducing non-fatal myocardial infarctions, but without reducing stroke rates or mortality [4]. However, this reduction is accompanied by an equally significant increase in relevant bleeding events, so that a net benefit appears questionable. These results coincided with follow-up studies, so that primary prophylactic treatment with acetylsalicylic acid should now be considered in the current ESC guidelines only in patients with diabetes and a high or very high risk of cardiovascular events and in the absence of contraindications (Table 1). In such cases, concomitant administration of a proton pump inhibitor should then be undertaken [6].
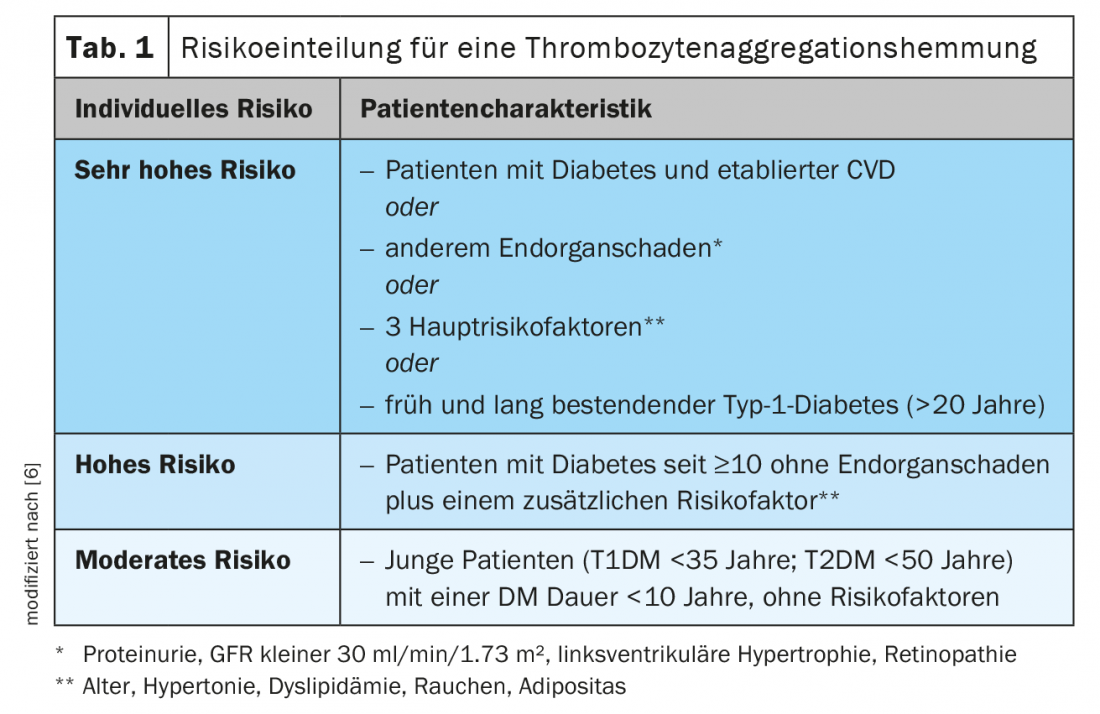
In secondary prophylaxis, as in the case report, the risk-benefit ratio of antiplatelet agents is clearly positive, so that they should be given mandatorily [6].
In patients with an acute coronary syndrome (ACS), the additional use of newer antiplatelet agents such as ticagrelor (Brilique®) or prasugrel (Efient®) is shown to be superior to treatment with clopidogrel in the first year after an acute coronary syndrome [7,8]. The choice of antiplatelet agents is analogous to that for nondiabetic patients and is based on the type of ACS. In high-risk patients (age >50 years and an additional risk factor such as age >60 years, type 2 diabetes with drug treatment, or chronic renal insufficiency with a creatinine clearance <60 ml/min/1.73m2), dual antiplatelet therapy can be given for longer than 12 months after myocardial infarction, if necessary [9]. In determining the duration of therapy and the products used, close and good interaction between the specialists involved and the general practitioner is important.
Antihyperglycemic therapy
Since 2006, the therapeutic palette for the treatment of hyperglycemia has expanded significantly. It is encouraging that all three newer forms of therapy, DPP4 inhibitors, GLP1 receptor agonists, and SGLT2 inhibitors, on the one hand, have no endogenous hypoglycemic potential, i.e., cannot trigger hypoglycemia themselves. On the other hand, they are weight neutral (DPP4 inhibitors) or may even be weight reducing (GLP1-RA > SGLT2 inhibitors) [10].
Initially and prior to drug treatment, the individual HbA1c target range is determined for each patient [11]. This is based, on the one hand, on the duration of the disease, the age and life expectancy of the patient, but also on the concomitant circumstances of the therapy. If there are no concomitant diseases and the patient’s motivation is high, a lower HbA1c target range of <6.5% can be aimed for or accepted if the medication to be used for this purpose has a low hypoglycemic risk or no endogenous hypoglycemic potential (Table 2) . If substances such as insulin or sulfonylureas are used, a target HbA1c range above 7.0% is sufficient.

In any case, however, drug therapy should be started early and, if necessary, also in combination, as this can lead to better long-term control than sequential antihyperglycemic therapy [12]. However, drug approaches should be complemented by appropriate basic education of the affected patient and adequate dietary and lifestyle modification, which, combined with weight reduction, sustainably improves diabetes control at any stage of the disease [10,11].
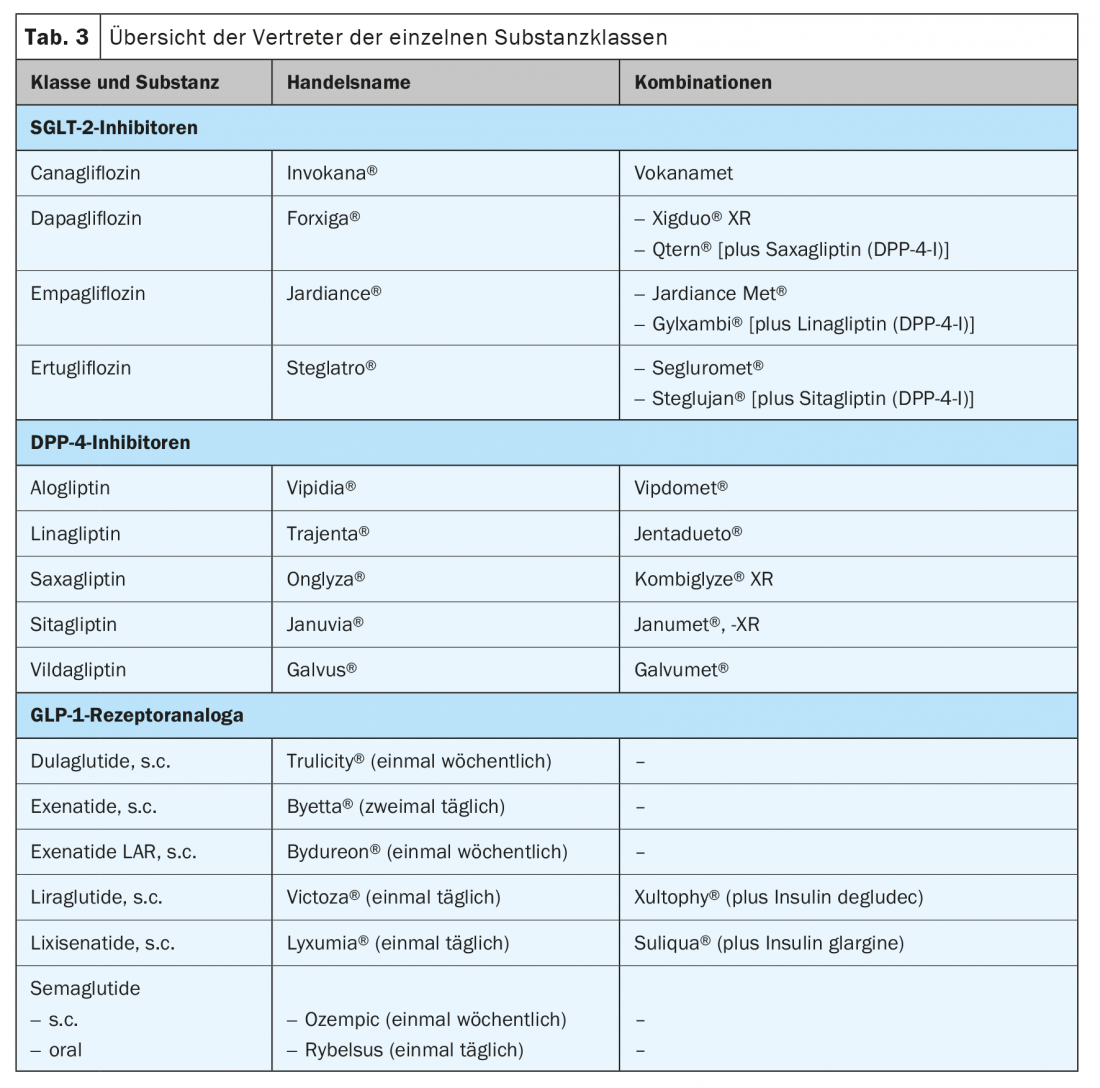
In the following, the three newer therapeutic classes will be discussed, as numerous scientific data have been collected on these in recent years, which help to characterize the substances in more detail and assist in the selection for the respective patient (Tab. 3).
Antihyperglycemic therapy – Dipeptidyl peptidase 4 inhibitors (DPP4 inhibitors):
The class of dipeptidyl peptidase (DPP)4 inhibitors now comprises five different substances (Table 3). The substances have approximately the same antihyperglycemic effect. With a baseline HbA1c of around 8%, a reduction of 0.5-0.7% can be expected. Furthermore, they are weight neutral [13]. They are usually very well tolerated by patients and can be used as one of the few substance classes in cases of impaired renal function [14]. In this case, the dosage of most preparations must be adjusted to the respective kidney function. Only linagliptin requires no further adjustment for renal insufficiency and is given at 5 mg per day. There is no effect on the preservation of renal function with the medication, but some reduction in the progression of albuminuria seems possible [15]. This substance class does not appear to have an effect beyond pure glucose lowering with regard to reducing the patient’s cardiovascular risk. The four endpoint studies published on the compounds show no reduction in event rates [16–19]. On the contrary, with saxagliptin, the rate of hospitalizations due to decompensated heart failure might even be slightly increased [19].
Because of its good tolerability, it is a medication that is ideal to use in elderly patients when glycemic control is the focus and another effect on the kidney and heart is less of a priority [20].
Antihyperglycemic therapy – sodium-glucose-linked transporter 2 inhibitors (SGLT2 inhibitors)
The youngest class of non-insulin therapeutics is the group of sodium-glucose-linked transporter (SGLT) 2 inhibitors (Table 3). This group of drugs exploits a physiological mechanism of sugar recovery in the kidney as a therapeutic principle. By blocking these transporters, much of the glucose previously freely filtered at the glomerulum is excreted in the urine. This results in a daily loss of about 60-70 g of glucose [21]. Thus, with a baseline HbA1c of 8%, one can expect an HbA1c reduction of 0.7-0.8%. In addition, weight is reduced by about 2-3 kg and blood pressure by 3-5 mmHg systolic. A particular advantage is good efficacy at any stage of the patient’s disease, since the mode of action does not depend on insulin secretion still being present or maintained. It can therefore be usefully used in early combination with metformin but also with insulin therapy. The main side effects are the increased incidence of genital fungal infections and some polyuria, which usually subsides during the course of treatment [21].
Administration is possible up to a renal function of 45 ml/min/1.73m2 (canagliflozin, dapagliflozin, empagliflozin) or 60 ml/min/1.73m2 (ertugliflozin), depending on the preparation [21]. Below that, the glucose-lowering effect is not appreciable. While the effect of the antihyperglycemic effect diminishes with decreasing renal function, this is not observed for the beneficial effects on the kidney or heart, which are still present even with decreased GFR [22].
In addition to glycemic effects, SGLT2 inhibitors appear to have particular effects in diabetic patients with renal disease and with heart failure and reduced pump function (HFrEF). With regard to renal effects, substances from this class of drugs appear to improve the kidney and preserve function at every stage. This is true in primary prevention, but also in secondary prevention, where albuminuria is favorably affected at any level of severity and renal function is also protected [23,24]. This has led to the inclusion of SGLT2 inhibition in the guidelines for the treatment of diabetic kidney disease, with a status equal to that of ACE inhibitors [25]. Interestingly, these effects are still present when renal function is already so severely impaired that no significant glucose lowering occurs. Canagliflozin is the first representative of the substance group to also demonstrate efficacy at a GFR <30 ml/min/1.73m2 in patients with albuminuria (ACR >300 mg/g) and can accordingly still be passed on here [22]. It is therefore exciting to ask to what extent the effects are also present independently of diabetes or diabetic nephropathy. Early results for dapagliflozin provide evidence that this may also be present in renal failure in nondiabetic patients [26].
The second domain independent of blood glucose lowering has emerged as the treatment of patients with diabetes and heart failure. Already in the first cardiovascular endpoint study, EMPAREG, which primarily studied diabetic patients with established prior cardiovascular disease, a significant 33% reduction in mortality was observed, which was accompanied by an equally strong reduction in heart failure events [23]. The effects on heart failure also seem to be a class effect and can thus be described by all four agents, whereas the reduction of cardiovascular mortality could not be demonstrated beyond doubt by all agents. Again, the question arises whether these effects on heart failure are independent of diabetes. Two studies are available for HFrEF that also demonstrated the same beneficial effect in the non-diabetes population [27,28]. Whether this is also the case in HFpEF, i.e. in heart failure with preserved systolic pump function, is the subject of current investigations and may be awaited with interest.
It should be emphasized that, based on these cardiac and renal data, the most recent treatment recommendations recommend the use of this medication in patients with established prior cardiovascular disease, heart failure, or renal problems regardless of HbA1c-value should be considered, i.e. even in patients who are actually already in the target range, e.g. with a monotherapy of metformin, an SGLT2 inhibitor should additionally be used.
Antihyperglycemic therapy – glucagon-like peptide 1 receptor agonists (GLP1-RA)
The strongest class of therapy in terms of blood glucose lowering, apart from insulin, is that of GLP1 recptor agonists. Until recently, representatives of this substance group were exclusively injected subcutaneously, although the frequency may vary from product to product. Since summer 2020, however, Rybelsus® (semaglutide) has also been available as the first representative that can be given as a tablet. When administering oral semaglutide, consideration must be given to ingestion modalities that allow the peptide to be absorbed and act in the first place. They are similar to those of thyroid preparations: min. 30 minutes before the first meal, without other medications or supplements with up to 120 ml of water after at least one meal. 8-hour fasting period.
The action of this group of substances is essentially via increased glucose-dependent secretion of insulin from pancreatic beta cells, combined with appetite inhibition via delayed gastric emptying and a direct effect on the central hunger center [29]. This also explains a good part of the observed side effects, which must rather be interpreted as an overshooting effect: Nausea up to and including vomiting. This is less with slow dose escalation and often decreases with therapy duration [29]. Depending on the initial HbA1c, reductions of between 0.8 and 1.6% can be expected, although individual differences are quite pronounced. This also applies to the effect on body weight, which decreases by 2-6 kg under therapy. Further beneficial effects result from a reduction in blood pressure and lipids [29]. In principle, four preparations are more widely used in Switzerland: liraglutide, exenatide LAR, dulaglutide and semaglutide. Which preparation should now be used can depend on the patient’s preferences (daily application vs. once-weekly administration; oral vs. subcutaneous administration). In cardiovascular high-risk patients with established atherosclerosis, a beneficial effect can be assumed for all four agents, with the best evidence for liraglutide and dulaglutide [21,30].
For this substance class, too, use independent of the HbA1c value is recommended in patients with known cardiovascular disease or renal problems.
In patients with renal insufficiency, these substances can also be given. They are safe to use and appear to slow progression somewhat in impaired renal insufficiency. In patients with a GFR <30 ml/min/1.73m2, however, the medication should be discontinued in the event of increased vomiting, as volume depletion in this case could conceivably lead to deterioration to the point of acute renal failure.
Antihypertensive therapy
Arterial hypertension is one of the most common comorbidities of diabetes, affecting more than 60% [31]. In a meta-analysis, blood pressure lowering of more than 10 mmHg in type 2 diabetics was shown to significantly reduce mortality (RR 0.87, 95% CI 0.78-0.96), cardiovascular events (RR 0.89, 95% CI 0.83-0.95), and stroke in particular (RR 0.73, 95% CI 0.64-0.83) [31]. At the same time, long-term microvascular complications such as retinopathy and albuminuria were also significantly reduced. On the other hand, there is heterogeneity of effects when blood pressure is lowered to systolic below 130 mmHg, because then only the risk of stroke is further reduced, with no additional benefit on cardiac or the microvascular end points. On the contrary, too aggressive lowering increases the risk of severe side effects [31]. It is recommended to treat arterial hypertension in diabetics with medication from a blood pressure of >140/90 mmHg. Target values of systolic below 130 mmHg are to be aimed for, but not below 120 mmHg. For age >65 years, a moderately higher target range >130-139 mmHg should be selected. Diastolic target range was defined below 80 mmHg but not below 70 mmHg (Table 4) [6]. As usual, the basis of any treatment is lifestyle modification. Drug treatment should primarily start with a combination therapy. Patients with diabetes benefit from ACE inhibitors and angiotensin-1 receptor antagonists, which should be used especially in cases of microalbuminuria, albuminuria, proteinuria, or LV hypertrophy [6]. Good combination partners are calcium antagonists or thiazide (like) diuretics. Promoting compliance can be achieved by using 3-combination in one pill, which are available in ever increasing numbers. It should be emphasized that blood pressure can once again be favorably influenced by the appropriate choice of antidiabetic drug. With the use of a GLP1 receptor agonist, such as liraglutide, a reduction of 1-2 mmHg or an SGLT-2 inhibitor, such as empagliflozin, of 4 mmHg can be observed [23,30].
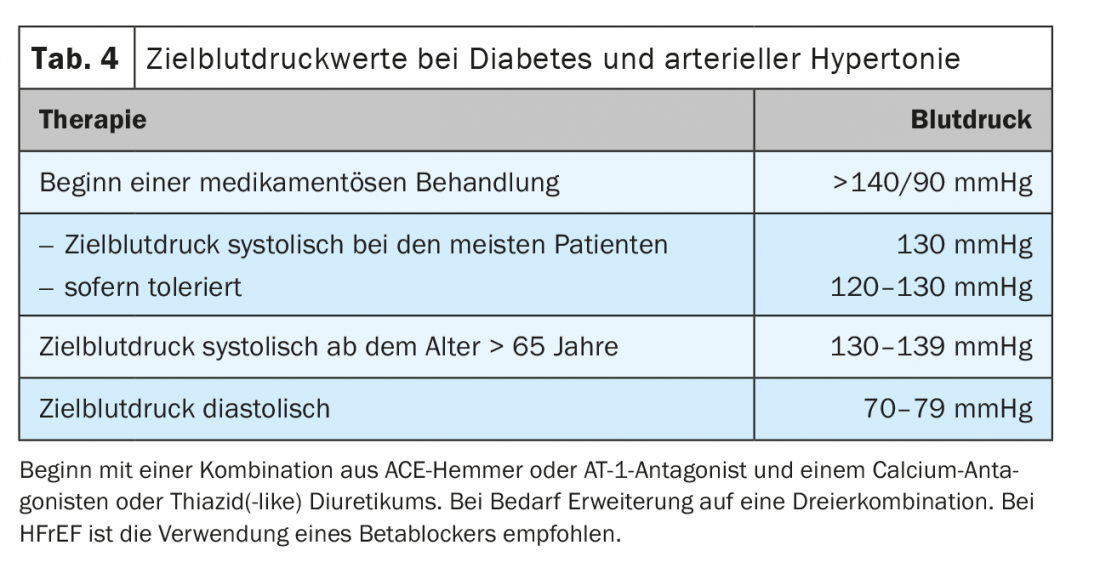
If there is concomitant heart failure with reduced pump function (HFrEF), the use of a beta-blocker is also recommended in diabetic patients to reduce mortality [6]. The combination of sacubitril and valsartan (Entresto®) also significantly reduced the primary endpoint of mortality or first hospitalization due to worsening heart failure in the diabetic subgroup, although to a slightly lesser extent than in non-diabetics. In patients with HFrEF, care should be taken to maximize the possible and tolerable dosage of heart failure medication and not to limit oneself by the blood pressure targets mentioned above.
Cholesterol lowering therapy
Study data over the past five years impressively show that with further lowering of LDL cholesterol, residual cardiovascular risk can be reduced. This makes sense especially if the patient’s risk is correspondingly increased compared to the general population. Most patients with diabetes are considered to be at high risk per se with regard to cholesterol-lowering therapy, for whom a target range of <1.8 mmol/l should now be aimed for according to the current guidelines. Only in a small group of diabetics with a moderate risk is a LDL target range <2.6 mmol/l adequate (Fig. 1) . In this situation, there is no need to calculate the individual risk with the otherwise so valuable AGLA calculator [32].
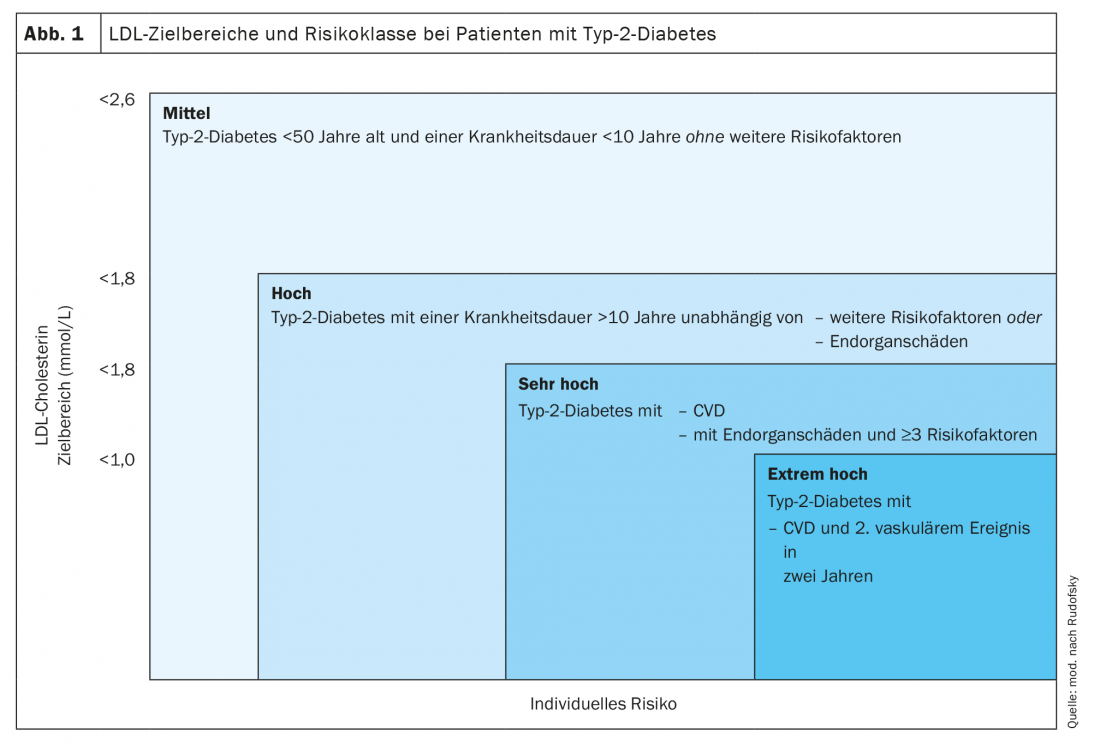
If diabetes is combined with manifest atherosclerotic disease, the risk increases to “very high” and a target LDL range <1.4 mmol/l should be aimed for. If a second vascular event occurs within 2 years on maximally tolerated statin therapy, the patient’s risk appears to be extremely increased. Further LDL lowering to a range <1.0 mmol/l is recommended. A second atherosclerotic event does not include an in-stent stenosis that requires treatment.
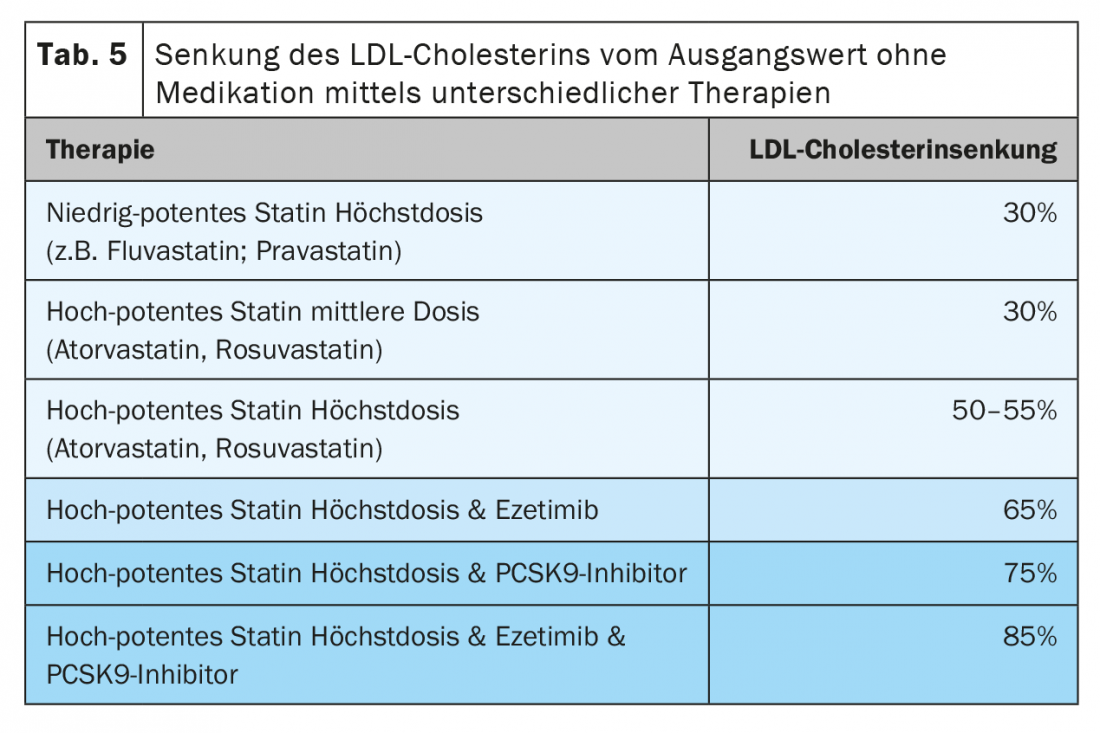
The first step in therapy is the administration of a highly potent statin (rosuva- or atorvastatin). In order to reach the target range, a high dosage is often necessary. However, our recommendation is still to start with a low dose (e.g. 10 or 20 mg), as below this the rate of side effects is lower and yet most of the LDL lowering is achieved. In a second step, the dosing out should then take place. This prevents a patient from being misclassified as statin intolerant due to intolerance of higher doses, which complicates the further course of therapy.
Nevertheless, if side effects occur with atorva and rosuvastatin, the use of fluva or pravastatin, which are less potent but often more tolerable, may also be appropriate in this risk population.
A further lowering of LDL is achieved by combination with ezetimibe, which is now also available in fixed combinations with both highly potent statins. This can achieve reductions of approximately 65% from the initial baseline. If this combination is dosed out, about 80% of patients can be brought into the target range. As a final escalation step, PCSK-9 inhibitors are then available, which achieve a further reduction of 50-60% from the LDL level already achieved under the combination (Table 5) [32].
Conclusion
In summary, the care of patients with diabetes, especially when arteriosclerotic disease has occurred, is a challenge in everyday clinical practice, since different therapeutic facets must be kept in mind and, if necessary, the interaction of different disciplines must be coordinated and agreed upon in order to ensure the best possible care for these high-risk patients. Through this article, we have attempted to cover the various topics, which by its nature can only be done in a cursory manner.
Take-Home Messages
- Primary prevention with acetylsalicylic acid should be considered only in patients with diabetes and high or even very high risk for cardiovascular events. On the other hand, secondary treatment with acetylsalicylic acid is mandatory.
- New therapies for hyperglycemia include: DPP4 inhibitors, GLP1 receptor antagonists, and SGLT2 inhibitors. All three classes of compounds have no hypoglycemic potential and are weight-neutral to weight-reducing.
- DPP4 inhibitors are particularly well suited for elderly patients without heart failure due to their good tolerability and applicability in impaired renal function.
- SGLT2 inhibitors represent the therapeutic of choice in diabetics with impaired renal function and/or heart failure with impaired pump function.
- GLP1-RAs have the most potent blood glucose-lowering effect, contribute to weight loss, and have a favorable prognosis in high-risk cardiovascular patients.
- Antihypertensive treatment is recommended in diabetes starting at a blood pressure of >140/80 mmHg with a target blood pressure of <130/80 mmHg.
- Target values for LDL cholesterol in patients with diabetes depend on overall risk profile and are defined from <2.6 to <1.0 mmol/l.
Literature:
- Almdal T, et al: The independent effect of type 2 diabetes mellitus on ischemic heart disease, stroke, and death: a population-based study of 13,000 men and women with 20 years of follow-up. Arch Intern Med 2004; 164: 1422-1426.
- International Diabetes Federation: IDF diabetes atlas 9th edition 2019. www.diabetesatlas.org
- Wong K, et al: Comparison of demographic factors and cardiovascular risk factor control among U.S. adults with type 2 diabetes by insulin treatment classification. J Diabetes Complications 2012; 26: 169-174.
- Baigent C, Blackwell L, Collins R, et al: Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009;373: 1849-1860.
- ASCEND Study Collaborative Group, Bowman L, Mafham M, Wallendszus K, et al: Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med 2018;379: 1529-1539.
- Cosentino F, Grant PJ, Aboyans V, et al: ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD 2019 European Heart Journal (2020) 41: 255-323.
- PLATO Investigators, Wallentin L, Becker RC, Budaj A, et al: Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009;361: 1045-1057.
- TRITON-TIMI 38 Investigators, Wiviott SD, Braunwald E, McCabe CH, et al: Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357: 2001-2015.
- PEGASUS-TIMI 54 Steering Committee and Investigators, Bonaca MP, Bhatt DL, Cohen M, et al: Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med 2015; 372: 1791-1800.
- Lehmann R, Gastaldi G, Czock A, et al: Swiss Recommendations of the Society for Endocrinology and Diabetes (SGED/SSED) for the Treatment of Type 2 Diabetes Mellitus (2020).
- Buse JB, Wexler DJ, Tsapas A, et al: 2019 update to: Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2020 Feb;63(2): 221-228.
- VERIFY study group; Matthews DR, Paldánius PM, Proot P, et al: Glycaemic durability of an early combination therapy with vildagliptin and metformin versus sequential metformin monotherapy in newly diagnosed type 2 diabetes (VERIFY): a 5-year, multicentre, randomised, double-blind trial Lancet. 2019 Oct 26; 394(10208): 1519-1529.
- Craddy P, Palin HJ, Johnson KI: Comparative effectiveness of dipeptidyl peptidase-4 inhibitors in type 2 diabetes: a systematic review and mixed treatment comparison. Diabetes Ther 5: 1-41, 2014.
- Davis TM: Dipeptidyl peptidase-4 inhibitors: pharmacokinetics, efficacy, tolerability and safety in renal impairment. Diabetes Obes Metab 16: 891-899, 2014.
- CARMELINA investigators; Perkovic V, Toto R, Cooper ME, et al: Effects of Linagliptin on Cardiovascular and Kidney Outcomes in People With Normal and Reduced Kidney Function: Secondary Analysis of the CARMELINA Randomized Trial. Diabetes Care 2020 Aug;43(8): 1803-1812.
- Green JB, Bethel MA, Armstrong PW, et al: Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015 Jul 16;373(3): 232-242.
- EXAMINE Investigators; White WB, Cannon CP, Heller SR, et al: Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 369: 1327-1335, 2013.
- CARMELINA Investigators; Rosenstock J, Perkovic V, Johansen OE, et al: Effect of Linagliptin vs Placebo on Major Cardiovascular Events in Adults With Type 2 Diabetes and High Cardiovascular and Renal Risk: The CARMELINA Randomized Clinical Trial JAMA 321: 69-79, 2019.
- SAVOR-TIMI 53 Steering Committee and Investigators; Scirica BM, Bhatt DL, Braunwald E, et al: Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 369: 1317-1326, 2013.
- Avogaro A, Dardano A, de Kreutzenberg SV, Del Prato S: Dipeptidyl peptidase-4 inhibitors can minimize the hypoglycaemic burden and enhance safety in elderly people with diabetes. Diabetes Obes Metab 17: 107-115, 2015.
- Scheen AJ: Sodium-glucose cotransporter type 2 inhibitors for the treatment of type 2 diabetes mellitus. Nat Rev Endocrinol 16: 556-577, 2020.
- CREDENCE Trial Investigators; Perkovic V, Jardine MJ, Neal B, et al: Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med 380:2 295-2306, 2019.
- Zinman B, Wanner C, Lachin JM, et al: Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015 Nov 26;373(22): 2117-2128.
- DECLARE-TIMI 58 Investigators; Wiviott SD, Raz I, Bonaca MP, et al: Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2019 Jan 24;380(4): 347-357.
- Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease.Kidney Int. 2020 Oct; 98(4S): S1-S115.
- DAPA-CKD Trial Committees and Investigators; Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al: Dapagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2020 Oct 8;383(15): 1436-1446.
- DAPA-HF Trial Committees and Investigators; McMurray JJV, Solomon SD, Inzucchi SE, et al: Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2019 Nov 21;381(21): 1995-2008.
- EMPEROR-Reduced Trial Investigators; Packer M, Anker SD, Butler J, et al: Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med. 2020 Oct 8;383(15): 1413-1424.
- Davidson JA: Differential effects of prandial and non-prandial GLP-1 receptor agonists in type 2 diabetes therapy.Postgrad Med 127: 827-841, 2015.
- Marso SP, Daniels GH, Brown-Frandsen K, et al: Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2016 Jul 28;375(4): 311-322.
- Emdin CA, Rahimi K, Neal B, et al: Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA 2015;313: 603-615.
- Authors/Task Force Members; ESC Committee for Practice Guidelines (CPG); ESC National Cardiac Societies. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Atherosclerosis. 2019 Nov; 290: 140-205.
HAUSARZT PRAXIS 2021; 16(7): 10-16