Today, the indication for cataract surgery is given in case of relevant subjective complaints and/or objective functional limitations caused by the cataract. Today, cataract surgery is performed in most cases on an outpatient basis. Oral anticoagulation usually does not need to be interrupted for surgery. Today, modern lens systems can allow a spectacle-free life in many cases if no other pathologies are present. The intraocular lens to be implanted must be individually adapted to the morphometry of the eye and the patient’s wishes, which requires extensive diagnostics and intensive consultation. For the past few years, femtosecond laser-assisted cataract surgery has been available, in which a computer-controlled laser performs certain steps of the surgery. The extent to which the advantages of this method outweigh its disadvantages is still the subject of intense debate.
The natural lens poses a special challenge for nature regarding total optical transparency and precisely defined refractive power. This is not an amorphous clear structure, as one might suspect, but a cellular association. Therefore, in the course of life, structural changes of the non-regenerative cells, protein denaturation and accumulation of degradation products lead to continuous opacification in the lens. Thus, it is a natural aging process that can be accelerated by pathological influences or even provoked early in life. Cataract surgery is already known from ancient Egypt. By the Middle Ages, the technique of cataract piercing, in which a needle was inserted into the anterior segments of the eye and the lens was pushed backward into the vitreous cavity to expose the optical axis, was already a widely used surgical method. Since then, cataract surgery has evolved rapidly.
Symptoms of cataract and indication for surgery
As a rule, the development of age-related cataract leads to a hardening of the lens nucleus (“nuclear sclerosis”) and thus to an increased refractive power of the lens resulting in myopia. Previously presbyopic patients are suddenly able to see near better or read again, and hyperopic patients find that distance vision is improved for them without spectacle correction. As the disease progresses, symptoms such as glare due to increased light scattering, loss of color perception, especially in the blue-green range, and loss of visual acuity develop. Due to the very slow progression of the process and the habituation effect over time, many patients do not notice this until they lose the ability to read. This is of particular importance because, for example, driving a motor vehicle is subject to higher requirements, and loss in this area is often not properly perceived by the patient.
Recent developments in cataract surgery allow routine, minimally invasive surgery with self-sealing wounds, which today allow rapid rehabilitation of patients within a few days and have reduced the surgical risk to a minimum. Accordingly, the indication for surgery has changed over the years. The term “mature cataract” no longer exists today. The decisive factor for the indication of surgery is the subjective and/or objective disturbance of the patient by the opacification of the lens. Since the development of multifocal lens systems, the exchange of the clear lens is now also offered for presbyopic patients in order to achieve spectacle independence. It is important to note here that the latter cases are “lifestyle” surgery, which should not be covered by health insurance.
Surgical Technology
Today, cataract surgery is a typical outpatient procedure. Usually, topical anesthesia (drip anesthesia) is used. In addition, because surgery is performed in nonperfused tissue, discontinuation of anticoagulation is not normally required and patients do not usually need to fast for surgery.
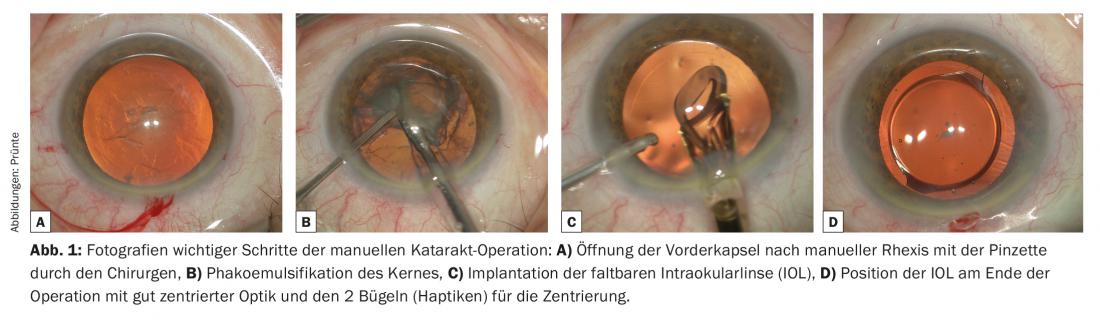
After disinfection, sterile draping and insertion of an eyelid holder, two to three small self-sealing valve incisions are made in the area of the limbus [1]. The main incision for removing the cloudy lens content and inserting the flexible intraocular lens (IOL) into the capsular bag is about 2.2 mm here. One to two paracenteses of less than one millimeter are required for auxiliary instruments such as spatulas, cannulas, etc. Due to the stability of the incisions even when pressure is applied to the eye, suture closure is not necessary. In a further step, an approximately 5.5 mm round opening of the anterior capsule (Fig. 1A) is created using a needle or forceps (capsulorhexis) and the lens mass is separated from the capsule with a liquid wave. This is followed by phacoemulsification of the core (Fig. 1B), the fragmentation of the hard core masses with simultaneous suction. The mechanism of action corresponds to that of an extremely fast vibrating tiny jackhammer. The volume deficit is continuously replaced by fluid flowing coaxially along the phaco tip from an infusion bottle. Subsequently, the lens capsule is polished from the inside by means of hydrojet and the remaining bark residues are suctioned off. This is followed by implantation of the IOL, which is usually foldable, with the aid of an injector system. (Fig.1C). Today’s IOLs consist of an approximately 6 mm optics and so-called haptics, small brackets that center the optics in the optical path of the eye by bracing it in the capsular bag. (Fig. 1D). Subsequently, viscoelastic excipients, which can be injected during surgery to stabilize the eye, are aspirated and the volume is replaced with fluid. This tones the bulb and thereby closes the valve incisions. Today, this method is extremely precise and safe for a skilled surgeon. Accordingly, full general rehabilitation is ensured immediately after surgery and visual rehabilitation within a few days.
Femtosecond laser-assisted cataract surgery (FLACS).
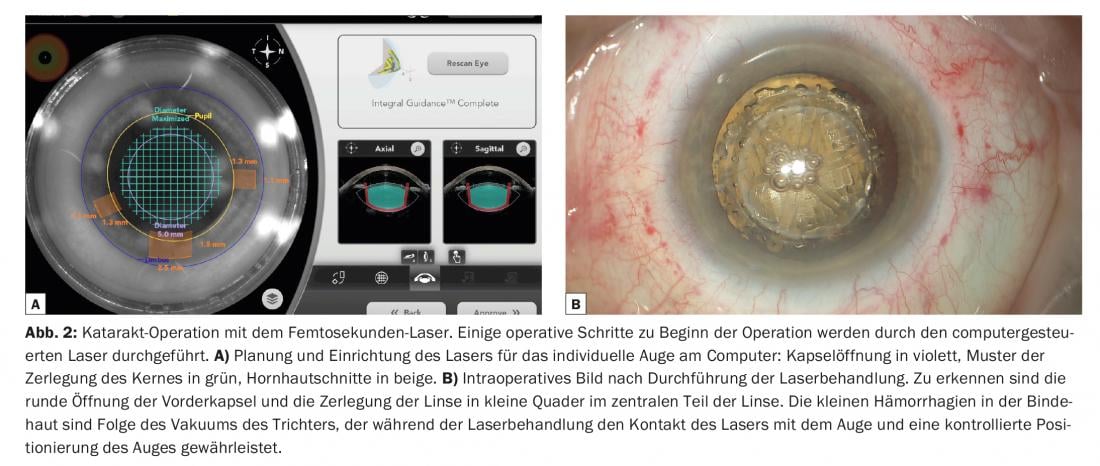
A laser-assisted method for cataract surgery has also been available for a few years. For cost reasons, this is now limited to the premium segment with corresponding co-payment by the patient. These lasers set ultrashort microexplosions, which can create cuts when placed close together similar to a stamp perforation. Accordingly, the use of the laser is limited to opening the capsule (rhexis), dissecting the lens nucleus into preselected shapes, and making corneal incisions. For the actual treatment, the eye must be docked to a vacuum system, through which a stable connection to the imaging system as well as to the laser is ensured. The advantage of this system is the great precision and reproducibility, as well as the virtually arbitrary variability of the design. The procedure is planned for each individual patient on the computer based on the imaging of the system (Fig. 2A) . After laser treatment, the pre-cut core parts (Fig. 2B) can be aspirated with reduced energy for disintegration and the IOL can be inserted with the usual surgical procedure.
A problem of FLACS is still the stringing together of small microexplosions to produce a cut and the resulting discontinuities of the cut edge. These can be a predilection site for uncontrolled rupture during surgery, especially in the area of the lens capsule (Fig. 3). According to initial publications, the rate of capsular rupture in laser-assisted surgery is 2%, about twice as high as in the manual technique. The extent to which the precision of a round capsule opening with a precisely defined diameter outweighs this is still the subject of intense debate [2].

At the moment, the high cost of acquisition and maintenance and the associated need for amortization through high additional costs for patients represent a certain obstacle to the spread of these systems. However, the development of improved and less expensive systems and the fact that many surgeons no longer learn the manual technique when using FLACS may promote its spread in the future [3].
Intraocular lenses (IOL)
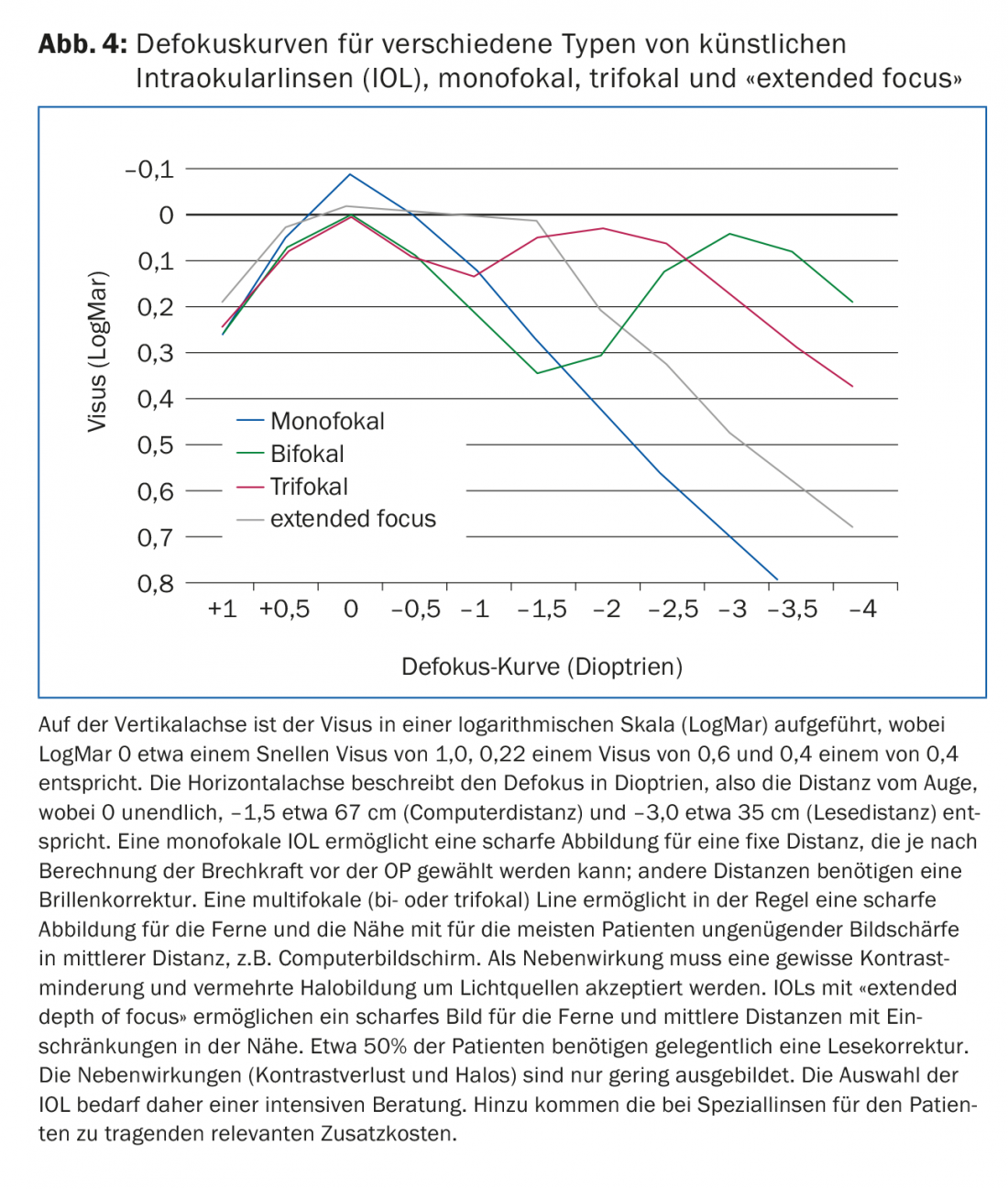
Today’s basic IOL is a lens with spherical optics. This has also been defined by the health insurance companies as the basis for payment. This also includes various tints of the IOL, so-called blue light filter lenses. Due to experimental results showing that blue light can damage the retina, lenses with an appropriate light filter and thus a slight yellow tint have been propagated for many years to protect the macula. In recent years, there have also been various developments in lens technology (Fig. 4).
Aspherical intraocular lenses: With increasing age, the human cornea exhibits a so-called positive spherical aberration, which causes light rays incident through the peripheral cornea not to be imaged in the same focus as centrally incident light rays. Since standard spherical lenses produce an additional positive aberration, this leads to a certain degree of contrast loss and night myopia. For this reason, aspheric intraocular lenses [4] have been developed and are offered as aberration-neutral aspheric IOLs or aberration-correcting aspheric IOLs. In principle, this optimizes the imaging quality of the eye. A problem here, however, is the individually very different asphericity of each eye. Therefore, for ideal IOL selection, individual asphericity should be determined preoperatively by topography examination. Due to this increased effort and the more complex production, additional costs arise which are usually not covered by the health insurance companies.
Multifocal IOLs: Restoration of accommodation with adaptation of the refractive power to the distance of the object to be observed is the goal of many years of research. Although such lenses are offered on the market, there is still no system that really satisfactorily implements these requirements [5,6]. Above all, biological problems such as fibrosis of the lens capsule with shrinkage of the capsular bag and proliferation and migration of equatorial lens epithelial cells pose an unsolved problem here. In the short term this leads to a loss of elasticity and in the long term to a loss of transparency of the lens-capsular bag complex.
Multifocal lenses (MIOLs) focus objects at two (bifocal MIOLs) or three (trifocal MIOLs) different distances. The patient learns to focus on the sharpest image over time. Basically, several images are projected simultaneously in different foci, which leads to so-called photic phenomena (glare & halo) and basically to a loss of contrast, especially at night. In most cases, neuroadaptation sets in over time, leading to some habituation in the patient. This can take several months. However, in some patients, this adaptation also fails to occur. In principle, these IOLs provide good distance and near vision, but the intermediate range is insufficiently supported for most patients, which is more pronounced with bifocal lenses than with trifocal lenses. A problem with these IOLs is corneal astigmatism, if present. Already from 0.5 to 1.0 diopters of astigmatism, the imaging quality is significantly limited, so that this must be minimized. This can be achieved on the one hand by an additional toricity (astigmatic optics to compensate a corneal astigmatism) of the multifocal IOL or also by a laser treatment of the cornea.
Extended depth of focus (EDOF) IOLs: These lenses create an extension of the focal point, resulting in an increased depth of field. In today’s popular model of this type of lens, there is also a correction for chromatic aberration, so that the loss of contrast is compensated and thus a sharp image is achieved from the distance range to a medium range of about 80 to 60 cm (typical computer distance). In addition, this principle achieves an extensive reduction of photic phenomena. In practice, these lenses make it possible to be largely independent of glasses, although this cannot always be guaranteed in the reading area.
Toric intraocular lenses: Toric IOLs allow correction of corneal astigmatism by toric optics of the IOL [7]. This must be precisely aligned with the axis of the astigmatism in the cornea. Even a slight deviation of only one degree leads to a loss of efficiency of 3.33%. This requires a correspondingly precise measurement and marking of the corneal axis as well as an exact adjustment of the lens in the capsular bag, which is not always unproblematic in a biological system. In addition, spontaneous rotations of the lens can still occur postoperatively, which in a few cases requires a post-rotation with a minor surgical procedure. In the presence of astigmatism, these IOLs have impressive potential to improve uncorrected visual acuity and therefore represent a distinctly useful, albeit expensive, option in lens surgery.
Prophylaxis of cataract
Although various pharmacologic agents and dietary supplements are still available today for the prophylaxis of cataract, none of these options has demonstrated proven efficacy in studies. However, a recent paper from China was able to show in animal and laboratory experiments that lanosterol can prevent and even reverse protein aggregations in the lens [8]. These are an important cause of the decrease in transparency of the aging lens. However, results on the application in the human eye are still pending. In principle, however, this could result in an extremely interesting approach, so we will certainly have to keep an eye on developments in this area.
Perioperative care
Today, cataract surgery is almost exclusively an outpatient procedure that can be performed under local anesthesia (usually topical anesthesia with eye drops). Nevertheless, this is a serious surgical procedure and a stressful perioperative situation for the patient. Accordingly, preoperative examination and documentation for the attention of the ophthalmologist and the supervising anesthesiologist is absolutely necessary. Thus, intraoperative systemic reactions, although rare, can be adequately responded to. In addition, conditions aggravating surgery can be identified preoperatively and taken into account in surgical planning. This includes, for example, therapy with alpha-1 receptor blockers, which lead to a so-called floppy iris syndrome, characterized by a narrow pupil and markedly mobile iris. Since cataract surgery is performed predominantly in non-perfused tissue (cornea, anterior chamber, lens), anticoagulation does not usually need to be suspended during normal cataract surgery.
Postoperatively, dressing is required under regular conditions only until the first postoperative day. This is followed by anti-inflammatory local therapy with topical steroids or non-steroidal anti-inflammatory drugs, which should usually be applied for two to four weeks. Due to the postoperative tissue reaction in the course of scar formation, there may be a relevant change in lens position and corneal refractive power within the first four to six weeks, so that definitive postoperative spectacle correction is usually not performed until four to six weeks after surgery. In almost all cases, the preoperative spectacle correction can no longer be used meaningfully after cataract surgery has been performed. In the immediate postoperative phase, this repeatedly leads to problems when driving a motor vehicle, as this is tied to minimal visual acuity. If necessary, the temporary adjustment of a correction in the first postoperative days, which then has to be re-determined and changed again in the further course, can be helpful here.
Otherwise, patients have few limitations postoperatively. Within the first week, there is a risk, albeit small, of septic endophthalmitis. Visits to swimming pools and saunas should therefore be avoided for about one week until the wound areas have finally epithelialized. Normal body washing or even showers, on the other hand, are allowed. Furthermore, no particular pressure should be applied to the eye for about one month in order not to endanger the self-sealing incisions. During this time, certain sports, such as contact sports, are not allowed.
Because cataract is a normal sign of aging, cataract surgery is the most commonly performed surgical procedure. Due to the advanced technical possibilities and the innovative lens systems, the result is extremely gratifying for the patient and for the attending ophthalmologist, if no other pathologies are present [9]. The development in the future will go in the direction of increasing standardization and thus also automation. This simplifies routine cataract surgery, especially for the surgeon. The extent to which these innovations will improve the postoperative outcome for the patient remains to be seen, at least in light of current developments. Special attention must also be paid to maintaining the surgeon’s ability to resolve complicated and more complex situations.
Literature:
- Zuberbühler B, et al: “Cataract Surgery”. 2008 Springer Heidelberg.
- Abell RG, et al: Femtosecond laser-assisted cataract surgery versus standard phacoemulsification cataract surgery: outcomes and safety in more than 4000 cases at a single center. J Cataract Refract Surg. 2015 Jan; 41(1): 47-52.
- Menapace RM, et al: Femtosecond lasers in cataract surgery: a critical review. Ophthalmologist. 2014; 111(7): 624-37.
- Pieh S: Aspherical intraocular lenses. Cataract Surgery, 2nd edition, chapter 7.4 ed. Gerd U. Auffarth, Publisher: Uni-Med, Bremen – London – Boston.
- Menapace R, et al: Accommodating intraocular lenses: a critical review of present and future concepts. Graefes Arch Clin Exp Ophthalmol. 2007 Apr; 245(4): 473-89. epub 2006 Aug 30. review.
- Koeppl C, et al: Pilocarpine-induced shift of an accommodating intraocular lens: AT-45 Crystalens. J Cataract Refract Surg. 2005 Jul; 31(7): 1290-7.
- Hayashi K, et al: Effect of astigmatism on visual acuity in eyes with a diffractive multifocal intraocular lens. J Cataract Refract Surg. 2010 Aug; 36(8): 1323-9.
- Ling Zhao, et al: Lanosterol reverses protein aggregation in cataracts. Nature 2015; 523: 607-611.
- Menapace R: Single-stage bilateral cataract surgery. Special supplement Ophtha 06/2015; 7-8.
HAUSARZT PRAXIS 2016; 11(10): 40-46