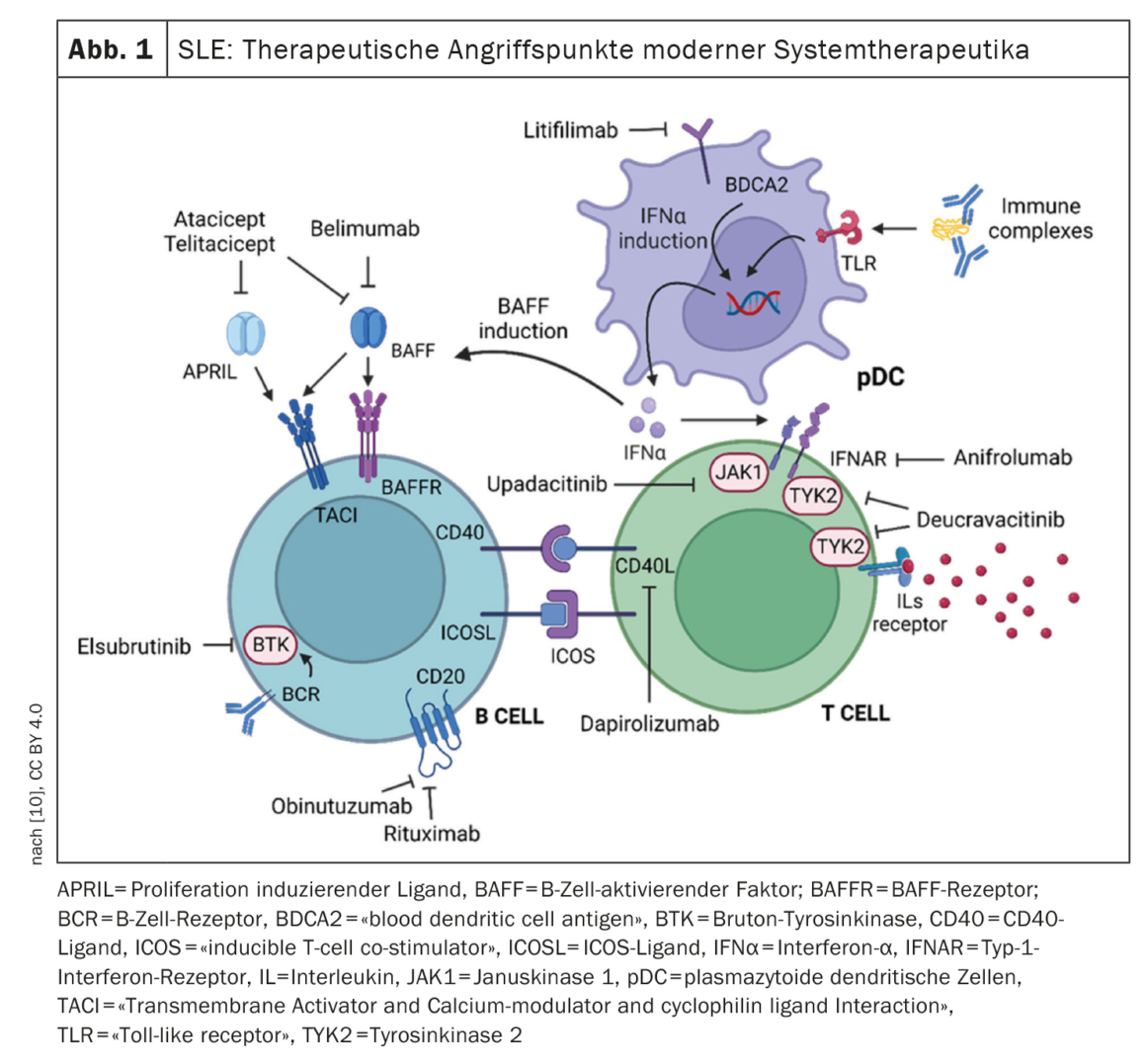
The multiple disease manifestations of systemic lupus erythematosus (SLE) are essentially a consequence of autoantibodies, immune complexes, and cytokines. New therapies specifically intervene in the immunopathogenesis of the complex autoimmune disease. For a long time, belimumab was the only biologic approved specifically for SLE. In the meantime, another monoclonal antibody, anifrolumab, has been approved for this indication, and numerous other drug candidates are currently being researched. With regard to a personalized treatment strategy, the identification of biomarkers is becoming increasingly important.
Given the expanded spectrum of treatment options, the focus is increasingly on identifying specific SLE phenotypes that respond to a particular therapeutic strategy, explained Prof. Martin Aringer, MD, University Hospital Dresden (D) [1]. According to the current EULAR/ACR classification criteria, the entry criterion for systemic lupus erythematosus (SLE) is the detection of positive antinuclear antibodies (ANA) [2,3,13]. Other immunologic parameters such as complement factors – serum complement levels (C3/C4) are often decreased in active disease – are also important, he said, but have lower sensitivity compared with ANA and are therefore simply part of the weighted criteria**. The latter are associated with domains associated with different SLE manifestations.
** Cut off value of 10 points
B cells and interferon signaling pathways in the focus of research
The etiopathogenesis of SLE has not been fully elucidated to date. But in the last decades, some mechanisms correlated with SLE have been identified [4]. These include in particular:
- Factors that contribute to B-cell activation or survival of autoreactive B cells
- Disturbances in clearance of apoptotic cellular material containing many SLE-associated autoantigens.
- chronic interferon influence as a “viral danger signal
It is assumed that pathogenic autoantibodies act in part directly on cells and can activate macrophages in the form of immune complexes and with the help of complement, which fuel the inflammatory response via proinflammatory cytokines. Furthermore, autoantibodies contribute to the survival of autoreactive lymphocytes and the formation of further autoantibodies via the B-cell cytokine BLyS/BAFF as well as interferons in a positive feedback loop [4]. Several new immunomodulatory drugs have been developed in recent decades. And it is hoped that large-scale “poly-omics” studies (genomics, proteomics and metabolomics) will provide further therapy-relevant insights into central immunological mechanisms [5].
Biologics as a beacon of hope – therapy selection moves to center stage
SLE is considered a B-cell mediated autoimmune disease. Rituximab is a B-cell depleting therapy that prevents the formation of plasmablasts. Despite evidence of efficacy in some studies, rituximab has not been officially approved in Switzerland in the indication SLE. However, upon request, the health insurance company can grant a cost approval [6]. The first biologic approved specifically for SLE was the BLyS inhibitor belimumab. By inhibiting BAFF/BLyS, belimumab causes selective blockade of B-cell activation. Under belimumab, the number of naïve and activated B cells and consequently plasmablasts is reduced, leading to a decrease in autoantibody levels and an improvement in C3 and C4 complement levels [7]. Biologics can catalyze the achievement of symptom reduction. But not only that, “Belimumab and anifromulab can help to phase out glucocorticoids and have been tested in different study populations,” said Professor Dimitrios T. Boumpas, MD, Department of Medicine, National and Kapodistrian University of Athens [8]. Anifrolumab was approved based on the results of the TULIP trials [9]. The efficacy of anifrolumab was shown to be positively correlated with high serum IFN levels [9]. Both belimumab and anifrolumab have been shown to be superior in placebo comparisons in SLE patients with norm deviant serologic markers at baseline (C3/C4 and/or anti-ds DNA). Belimumab has been tested in randomized trials in various populations. In addition to the already approved biologics belimumab and anifrolumab, there are promising Phase II data from iberdomide, litifilimab and deucravacitinib. In addition, various other drug candidates are being explored [10] (Fig. 1) .
Congress: EULAR Annual Meeting
Literature:
- “Lupus classification and diagnosis still based on clinical manifestations,” Prof. Dr. Martin Aringer, University Hospital Dresden, EULAR Annual Meeting, May 31-June 03, 2023.
- Aringer M, et al: 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol 2019; 71: 1400-1412.
- Aringer M, et al: 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis 2019; 78: 1151-1159.
- Aringer M, Finzel S, Voll RE: Immunopathogenesis of systemic lupus erythematosus. Z Rheumatol 2022. https://doi.org/10.1007/s00393-022-01214-4
- Aringer M, et al: A glimpse into the future of systemic lupus erythematosus. Ther Adv Musculoskelet Dis 2022; 14: 1759720X221086719.
- Magazine for Lupus Erythematosus Affected People, 1/2017; www.lupus-suisse.ch,(last accessed 06/23/2023).
- Hiepe F: New insights into the pathogenesis of SLE and their implications for the development of new therapeutic concepts. Act Rheumatol 2020; 45: 328-333.
- “How to treat Lupus,” Professor, Dimitrios T. Boumpas, MD, EULAR Annual Meeting, May 31-June 3, 2023.
- Morand EF, et al; TULIP-2 Trial Investigators. Trial of anifrolumab in Active Systemic Lupus Erythematosus. N Engl J Med 2020; 382(3): 211-221.
- Venturelli V, Isenberg DA: Targeted Therapy for SLE-What Works, What Doesn’t, What’s Next. Journal of Clinical Medicine 2023; 12(9): 3198.
www.mdpi.com/2077-0383/12/9/3198,(last accessed 23.06.2023) - Ruiz-Irastorza G, Gertsias G: Rheumatology (Oxford) 2020; 59(Suppl. 5): v69-v81.
- Choi ST, et al: Subscale analysis of quality of life in patients with systemic lupus erythematosus: association with depression, fatigue, disease activity and damage. Clin Exp Rheumatol 2012; 30: 665-672.
- Fanouriakis A, et al: 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus.Ann Rheum Disease 2019; 78: 736-745.
- Miyazaki C, et al: Treatment patterns and medical costs of systemic lupus erythematosus patients in Japan: a retrospective claims database study. J Med Econ 2020; 23: 786-799.
HAUSARZT PRAXIS 2023; 18(7): 32-33 (published 12.7.23, ahead of print).