With the increasing variety of personalized and immuno-oncological therapeutic options, the importance of predictive markers is also increasing. To date, tissue diagnostics, including invasive biopsy, are necessary to determine this. This could soon change with the introduction of so-called liquid biopsy. However, there are still a number of hurdles to overcome before large-scale deployment.
Where genetic analysis previously required the removal of tumor tissue, a simple blood sample could soon suffice. In the context of blood-based nucleic acid analysis using liquid biopsy , circulating tumor cells, tumor DNA and RNA are isolated from venous blood and examined. It is a method that is only made possible by the highly sensitive detection of nucleic acids. If this can be validated for widespread use, it could, among other things, significantly simplify the diagnosis of driver mutations and thus the choice of therapy. Although standardized processes and long-term data are currently still lacking, the technology has experienced a real boom over the last few years. This is reflected in an impressive increase in congress contributions and publications. If the PubMed search for “liquid biopsy” yields a meager 21 articles published in 2013, the number for 2020 is 1374 [1]. The new diagnostic field also appears to be economically interesting. As a result, a large number of companies have emerged that are dedicated exclusively to the research and marketing of Liquid Biopsy . The most famous of these may be the startup GRAIL, which is backed by Bill Gates and is particularly dedicated to early cancer detection using circulating cell-free tumor nucleic acids [2].
Tumor DNA in blood
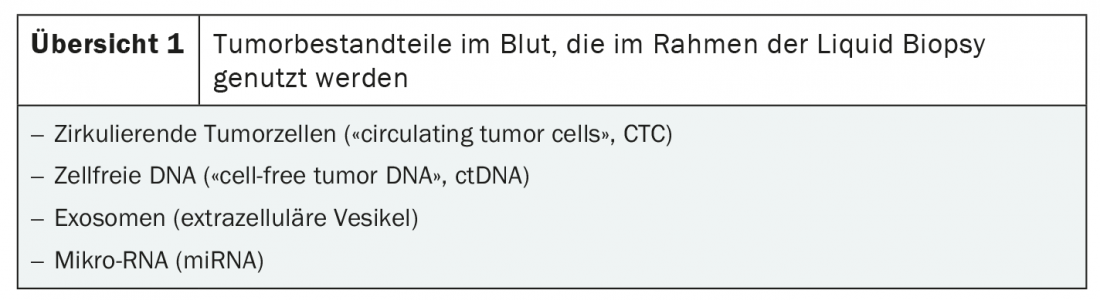
A prerequisite for liquid biopsy is the isolation of tumor components in the blood. There are two main sources of malignant DNA: circulating tumor cells (CTC) and cell-free tumor DNA (ctDNA). In addition, liquid biopsy can be used to identify tumoral exosomes and microRNA (miRNA) are detected (overview 1) [3]. The method is based on highly sensitive nucleic acid detection methods such as parallel sequencing (NGS), PCR and single cell analysis [3,4].
The different approaches are used in different areas based on their advantages and disadvantages. For example, tumor heterogeneity can be mapped by analyzing ctDNA, whereas predicting overall tumor composition using circulating tumor cells is more difficult. In both cases, a prognostic value of the examination could be shown for different malignancies: The more circulating tumor cells as well as cell-free DNA detected, the higher the risk of recurrence and progression. Furthermore, tumor grade and stage correlate with ctDNA load. But the diagnostic possibilities go far beyond such quantitative analyses. Qualitative changes are also of prognostic and predictive importance. Not only can they be used to identify therapeutic targets and genetic aberrations, but they also serve as progression parameters that could be used to assess therapeutic response in the future. For example, persistence of PD-L1 expression on circulating tumor cells under appropriate checkpoint blockade is considered to be prognostically unfavorable [5]. Similar findings apply to tyrosine kinase inhibitor therapy in lung cancer patients: In response, ctDNA levels for epidermal growth factor receptor (EGFR) mutations fall [6]. In general, microsatellite instability (MSI) in ctDNA, among others, seems to indicate advanced tumor stage. Many investigations in the field are currently in full swing, and with the increasing number of therapeutic targets and predictive markers, there are countless more questions awaiting suitability testing for liquid biopsy .
Of exosomes and micro RNA
However, not only circulating tumor cells and ctDNA are the subject of current research, but also other tumor components that can be detected in the blood: Exosomes and microRNA (miRNA). The latter are small, non-coding RNA fragments. These are involved in the regulation of proliferation, cell differentiation and apoptosis and thus function as oncogenes or tumor suppressor genes [7]. Clinically, miRNA is particularly promising as a biomarker, as the expression level correlates with malignant transformation. And often before phenotypic changes become detectable [8]. In addition, each malignancy has a characteristic miRNA signature – a big plus when it comes to determining the tissue origin of a poorly differentiated tumor. Whether in early detection or to clarify tumor identity, miRNA is likely to be heard from more in the coming years.
In this framework, an analysis of exosomes, i.e. extracellular vesicles, is also of interest. This is because these contain high concentrations of miRNA. Like presumably all cells, tumor cells secrete the vesicles of endosomal origin. Their role is still largely unclear, but is being intensively researched. For example, there is evidence that tumor exosomes can promote angiogenesis or, for example, inactivate therapeutic antibodies by expressing tumor antigens.
Whether ctDNA, circulating tumor cells, miRNA or exosomes – Liquid Biopsy can be considered for numerous application areas. In particular, the method could be ideally suited for early tumor detection, genetic characterization, and as a progression parameter under therapy [4]. The advantages are obvious: the less invasive diagnostic procedure, which is also possible in the absence of tumor tissue and, on top of that, is comparatively low-risk, means that a dynamic image of the genetic tumor profile can be obtained. On the one hand, recurrences can be detected early and, on the other hand, their often altered molecular profile can be deciphered by blood sampling – and attacked accordingly. Should liquid biopsy become established as a standard method, this would probably represent another milestone in the era of personalized therapy planning.
Further development necessary
Even if much research has already been done, the process is still in its infancy. Accordingly, there are still great uncertainties in the evaluation and also in the implementation. Applicability appears to differ significantly between tumor types and stages. For example, ctDNA is detectable in only about 70% of metastatic malignancies. In many tumor entities, a significant proportion of patients have almost no ctDNA. In brain tumors, the use of ctDNA analysis is questionable due to the blood-brain barrier, as very few DNA fragments even enter the blood [3,4]. With further increases in the sensitivity of the measurement methods, these limitations can probably be countered increasingly efficiently in the coming years. However, some technical optimizations still have to be made before it can be used in everyday clinical practice [4].
In order to enable a broader application, there is currently also a lack of standardization and quality management of the methods. Experts agree on this and call, among other things, for the definition of clear cut-off values and standardized panels. The extent to which the various markers are able to represent tumor heterogeneity also needs to be determined. The possibility of detecting molecular characteristics of a malignancy more completely than, for example, by means of a classical biopsy could represent a further decisive advantage of the new method. The importance of liquid biopsy in early cancer detection, with all its dilemmas, remains to be seen. In this setting, the relevance of a “pathologic” finding should be analyzed in detail, particularly with regard to the actual risk of disease, before broader use of diagnostics [4]. This means that there is still a long way to go before the technology is put into daily practice, and it will certainly remain exciting over the next few years.
Source: Wallesch M, Wirth M, Wollenberg B: “Liquid biopsy” as a key player in immuno-oncology. ENT. 2020; 68(12): 899-904.
Literature:
- www.ncbi.nlm.nih.gov/pubmed/ (accessed 03/07/2021)
- https://grail.com/ (accessed 07.03.2021)
- Nitz P: Liquid Biopsy. German Cancer Society; 2018. www.krebsgesellschaft.de/onko-internetportal/basis-informationen-krebs/diagnosemethoden/liquid-biopsy.html (accessed 07.03.2021)
- Dahl E: Diagnostics: Liquid Biopsy – Status 2016. Dtsch Arztbl. 2016; 113(4). DOI: 10.3238/PersOnko/2016.09.30.01
- Hofman P, et al: Liquid biopsy in the era of immuno-oncology: is it ready for prime-time use for cancer patients? Ann Oncol. 2019; 30(9): 1448-1459.
- Thress KS, et al: Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat Med 2015; 21(6): 560-562.
- Iorio MV, Croce CM: MicroRNA dysregulation in cancer: diagnostics, monitoring and therapeutics. A comprehensive review. EMBO Mol Med. 2017; 9(6): 852.
- du Rieu MC, et al: MicroRNA-21 is induced early in pancreatic ductal adenocarcinoma precursor lesions. Clin Chem. 2010; 56(4): 603-612.
InFo ONCOLOGY & HEMATOLOGY 2021; 9(2): 42-43.