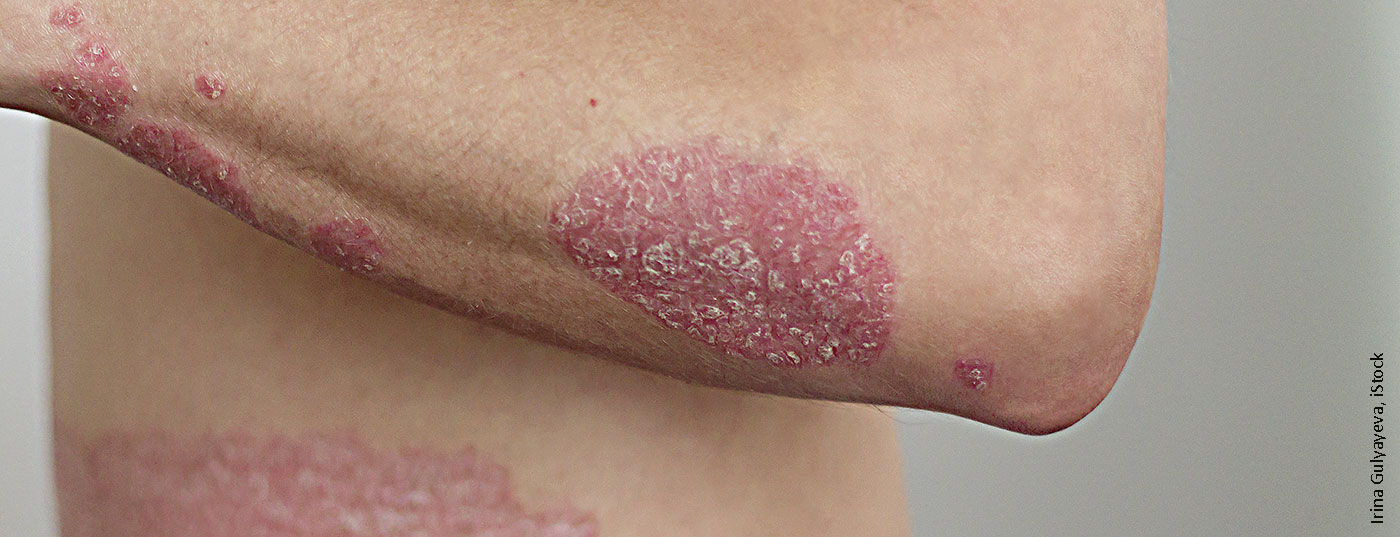
Psoriasis is nowadays well treatable by molecular therapies. Biologics enable optimized response rates. Compared to earlier representatives, new drugs of this substance class are characterized by sustained overlasting effects. This has been objectively proven several times.
Data from RCTs show that over 20 years response rates have improved from 16-25% to 90% PASI75 and nowadays PASI90 and PASI100 are realistic treatment targets [1,2]. The availability of targeted biologic therapies has been accompanied by an improved biological understanding of the pathogenesis of psoriasis, explained James G. Krueger, MD, PhD, Rockefeller University (USA). Approximately 15 years ago, it was recognized that inflammatory skin diseases are associated with T-cell subpopulations.
Paradigm shift of the pathogenetic explanatory model.
The IL17/IL23 pathogenesis model, which replaced earlier etiologic concepts in 2010, provides the following description of the cascade underlying psoriatic lesions [3]:
1) Dendritic cells produce IL23, whose outputs activate IL17, 2) IL17 triggers the production of cytokines and other inflammatory molecules by keratinocytes.
Data show that after two months of treatment with risankizumab (IL23 antagonist) a reduction of psoriasis-typical feed-forward mechanisms in the epidermis is measurable. After three months of therapy with secukinumab (IL17 antagonist), a high reduction of various IL17-related processes is detectable [2].
“Drug survival” critical to long-term strategy
Psoriasis is a lifelong disease – a key question is whether psoriasis symptoms can be effectively controlled over time using biologics. Whereas in the past the duration of treatment of psoriasis drugs was limited by toxicity, today the clinical benefit and the “drug survival” (duration of use) are decisive for this. In 2010, one of the first studies regarding durability of clinical benefits of biologics was published. Therein, it was shown that the response of a therapy with ustekinumab (IL12/IL23 antagonist) measurable at 12 and 24 weeks could be maintained over a period of 3 years, Dr. Krueger explained [1].
After another 5 years, registry data of biologics-naive patients were evaluated. With ustekinumab, the positive effects were maintained over a period of 4-5 years, in contrast to treatment with TNFα inhibitors, where there was a rapid decline in effects over the same period and only 50% of patients maintained therapy. This phenomenon may be due to different mechanisms of action, explains the speaker [1]. Drug survival” plays a decisive role here. This refers to the duration until a therapy is discontinued and, in addition to efficacy, is influenced, among other things, by the tolerability of a preparation.
|
Summary Achieving 100% clearance (PASI100) reflects psoriasis-related background activity present prior to treatment. In addition to PASI100 or PGA0 for as many patients as possible, another goal for the future is a reduction in “molecular scars” in remitted lesions. This, and the inclusion of disease-associated comorbidities in treatment, should enable a better quality of life. There are interesting differences in “molecular scars” in clinically remitted lesions across different biologics, with new generation agents being superior in this regard according to responder-to-responder analyses [1,4–6]. This also applies to the “drug survival” (duration of use), which plays an important role for long-term therapy strategies. |
“Molecular Scars” as an indicator of sustainable efficacy
Long-term data from RCTs and extension studies illustrate the sustained effects of modern biologics. Both the 5-year data of ustekinumab (IL12/IL23 antagonist) and brodalumab and ixekinumab (both IL17 antagonists) show stable durable effects in terms of PASI75/90/100 response rates. With regard to IL23 inhibitors alone, the data basis is smaller, since representatives of this substance class have not been around for very long. 4-year data of tildrazikumab show a similar course to IL17 antagonists. This is also true for 3-year data of guselkumab and 2-year data of risankizumab. So-called “molecular scars” are a measurable indicator of residual inflammatory activity that persists despite suppression of lesional processes. This is the remaining pathological gene expression profile (transcriptome genes with <75% improvement at the time of endpoint analysis). This contains residual inflammatory information that can reinitiate a psoriasis flare.
A randomized head-to-head study [4] showed that ustekinumab leads to a higher degree of remission at the molecular level compared to etanercept (TNFα inhibitor) (responder-to-responder analysis). There were also highly significant differences between risankizumab and ustekinumab [5]. Compared with ustekinumab, guselkumab showed a greater reduction in T cells and inflammatory dendritic cells, among other effects [6].
Source: EADV, Madrid
Literature:
- Krueger JG: Biologics in Psoriasis, Training and education forum, James G. Krueger, MD, PhD, Rockefeller University (USA), EADV Madrid, Oct 11, 2019.
- Krueger JG, et al: JACI 2019; 144(3): 750-763.
- Hawkes JE, Chan TC, Krueger JG: JACI 2017; 140(3): 645-653.
- Brodmerkel C, et al: JACI 2019; 143(5): 1965-1969.
- Visvanathan S, et al: JACI 2019; 143(6): 2158-2169.
- Li K, et al: www.psoriasisg2c.com, last accessed 10.01.2020.
DERMATOLOGIE PRAXIS 2020; 30(1): 20 (published 2/25/20, ahead of print).