Pulmonary embolectomy has become an important therapeutic option in patients with acute massive pulmonary embolism with present signs of right ventricular strain when the indication is carefully and interdisciplinary (cardiac surgery, cardiology, angiology, intensive care medicine, and anesthesiology). Our current algorithm at Inselspital for patients with acute pulmonary embolism is first to rapidly classify patients into risk groups so that if a “high-risk” or “intermediate-risk” situation is present, further therapeutic options (in addition to anticoagulation) can be evaluated as quickly as possible. Today, the results of surgical pulmonary embolectomy are very good and could be confirmed by data from different groups. Treatment of severe central pulmonary embolism should be performed in a center where surgical therapy is offered in addition to drug and catheter-based local lysis therapy.
Acute pulmonary embolism (LE) is an important disease of the cardiovascular system with an annual incidence of 100-200 per 100 000 persons [1]. The spectrum of pulmonary embolism ranges from clinically inapparent incidental findings on imaging to acute massive pulmonary embolism with cardiogenic shock and death.
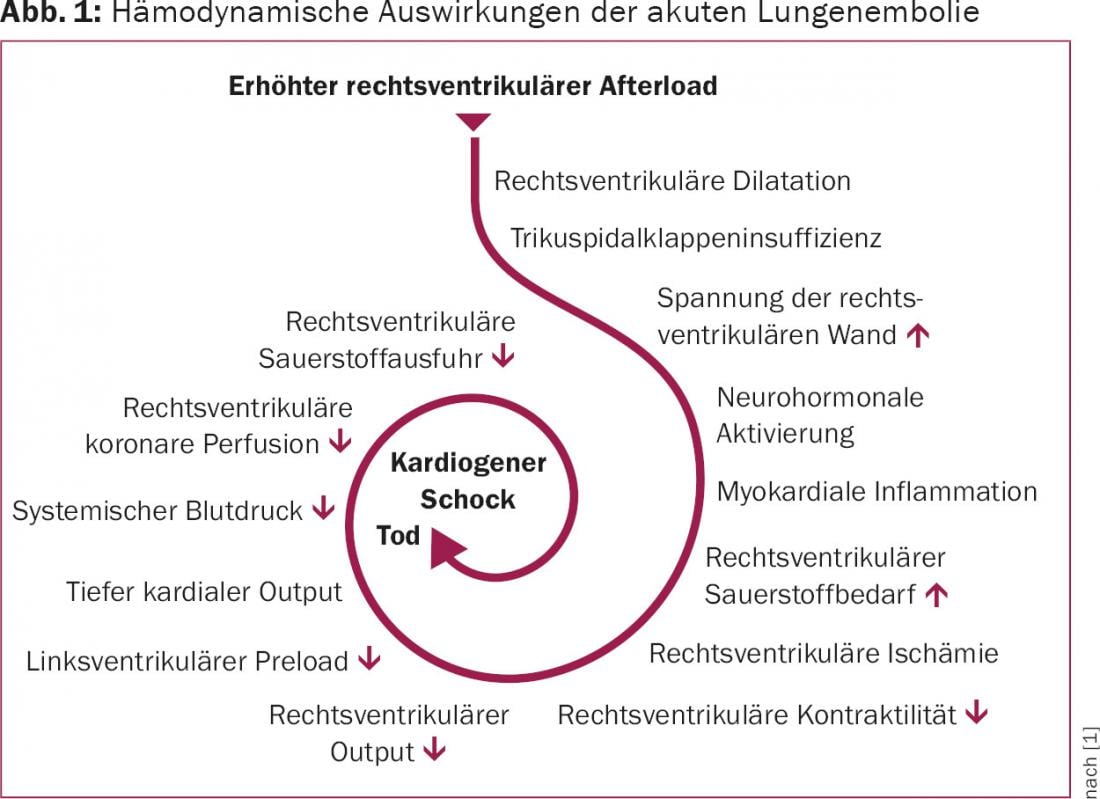
When %–50% of the entire pulmonary circulation is obstructed by thromboembolism, resistance increases, and pulmonary arterial pressure rises. With the associated immediate increase in afterload, the wall stress of the right ventricle increases and so does the oxygen demand, and relative right ventricular ischemia occurs, which in turn leads to a decrease in right ventricular output. This decrease consecutively reduces left ventricular cardiac function and may ultimately result in cardiogenic shock or death of the patient (Fig. 1).
New guidelines
In 2014, the European Society of Cardiology (ESC) published new guidelines for the diagnosis and treatment of acute pulmonary embolism [1]. According to the ESC, acute pulmonary embolism is classified according to the estimated mortality risk (“high-risk”, “intermediate-risk”, “low-risk”). This classification guides the decision on further therapy. The most essential factor in the prognosis of acute pulmonary embolism is right ventricular strain. A patient with clinical signs of right ventricular dysfunction such as persistent hypotension and cardiogenic shock has a high risk of mortality and is classified in the “high-risk” group.
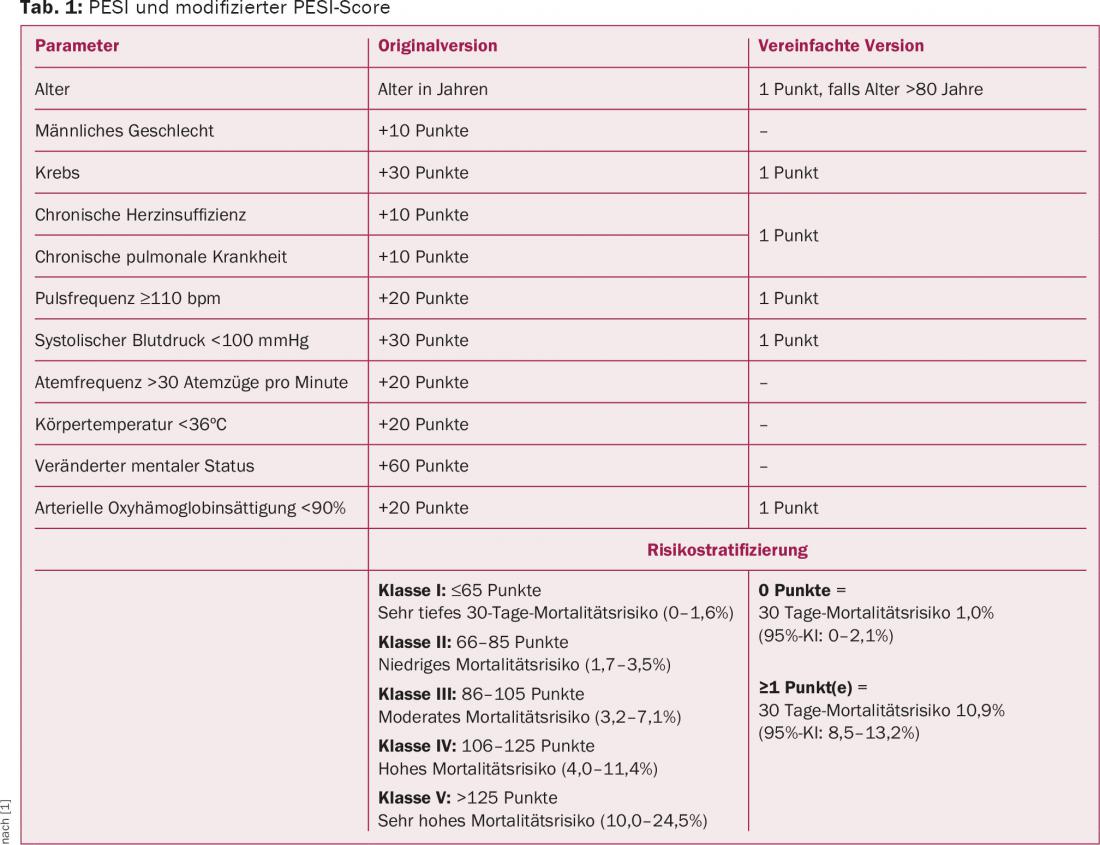
Using additional clinical parameters, the Pulmonary Embolism Severity Score Index (PESI) can be used to further differentiate the risk for patients with acute pulmonary embolism (Table 1) . In patients with a PESI class II-V, it is recommended to actively search for right ventricular strain by imaging and laboratory.
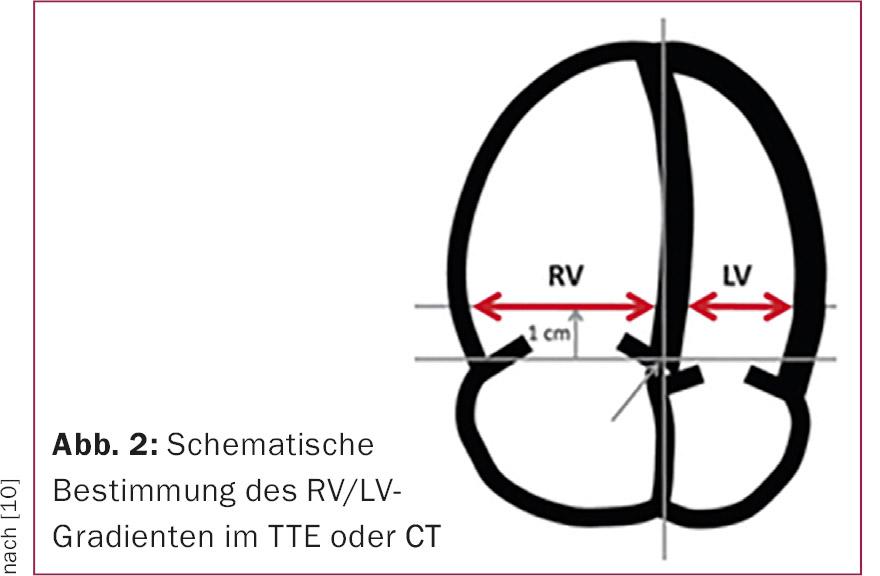
It is reasonable to assume that approximately one quarter of patients have echocardiographic evidence of right heart strain [2]. Dilation and/or hypokinesia of the right ventricle and an increase in the RV/LV ratio (Fig. 2) are indicative of right ventricular stress from acute pulmonary embolism. In addition, echocardiography can reveal a patent foramen ovale or intracavitary thrombi in the right atrium/ventricle, which influences the decision on further therapy.
In addition to imaging by transthoracic echocardiography (TTE) and computed tomography (CT), laboratory parameters may also provide evidence of right ventricular strain. Elevated troponin, reflecting cardiac damage in patients with acute pulmonary embolism, is associated with a worse prognosis [3].
Classification of patients
Based on these clinical, imaging, and laboratory parameters, patients with acute pulmonary embolism are classified (Fig. 3) . Patients with persistent systemic hypotension (defined as systolic pressure <90 mmHg or drop in systolic pressure >40 mmHg over 15 min) or cardiogenic shock are classified in the high-risk group. Patients without these clinical signs are further subdivided with imaging (TTE, CT) and laboratory parameters into “intermediate-risk” if they show signs of right ventricular strain, or “low-risk” if no clinical signs are detectable and there is no right ventricular strain on imaging or in the laboratory. Patients in the “low-risk” group have a very low mortality risk and are already sufficiently well treated with anticoagulation.
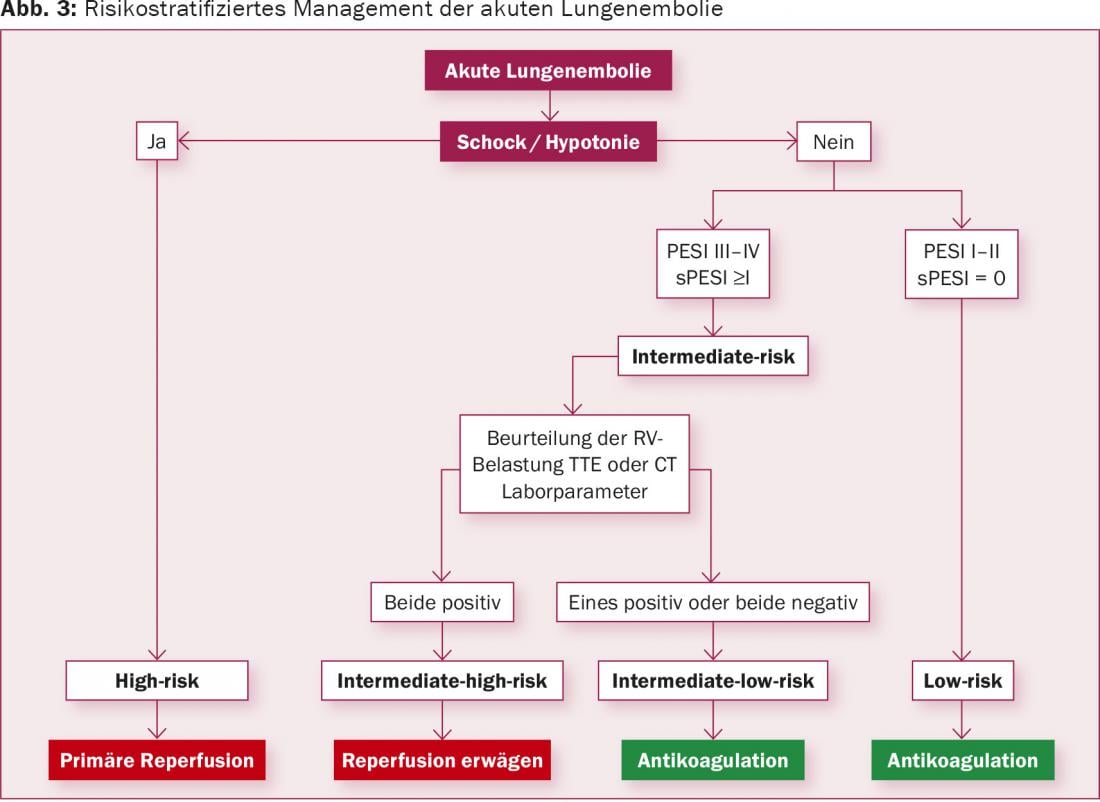
Reperfusion therapy is clearly indicated for patients in the “high-risk” group. Primarily, systemic fibrinolysis is recommended in the guidelines; alternatively, surgical pulmonary embolectomy or local catheter procedures should be evaluated. Local pharmacomechanical lysis therapy shows some advantages over systemic lysis in different applications in the current literature and is performed in our center as the therapy of choice for nonsurgical reperfusion therapy.
The situation is somewhat more differentiated for patients in the “intermediate-risk” group. Several studies have demonstrated that patients with signs of right heart strain and additional positive cardiac enzymes benefit from continuing therapy (systemic fibrinolysis) in addition to anticoagulation, as this reduced the rate of cardiac decompensation [4]. However, this advantage is limited with systemic fibrinolysis by a significantly increased rate of bleeding complications (particularly intracranial hemorrhage). However, it seems clear that certainly patients in the “high-risk” group and probably also patients in the “intermediate-risk” group benefit from reperfusion therapy in addition to anticoagulation. According to the current guidelines, systemic fribrinolysis is not generally recommended in intermediate-risk patients because of the risk of bleeding [1]. However, surgical embolectomy or catheter-based local fibrinolytic therapy should be evaluated in this patient population with a IIb recommendation [1].
The role of surgery in acute pulmonary embolism: earlier…
Before patients with acute pulmonary embolism could be effectively treated with drugs – anticoagulants were discovered and used clinically only later – the first successful surgical therapy of central massive pulmonary embolism was described [5]. In 1924, the first successful pulmonary embolectomy was performed. The surgical technique had been developed by Trendelenburg in the years before. Trendelenburg surgery has little in common with modern surgical therapy for pulmonary embolism. One has to imagine: The affected patients had to be taken to the operating room immediately after diagnosis of the acute pulmonary embolism and the circulatory collapse it triggered. This meant that the acute pulmonary embolism had to be observed by a specialist. Then, via thoracotomy, the pericardium was opened, the pulmonary artery was ligated, and thus an inflow occlusion was created. Subsequently, the pulmonary artery was opened and the thrombus mass was removed manually. It is clear that Trendelenburg surgery had a high perioperative mortality. The development of extracorporeal circulation revolutionized cardiac surgical options, and the procedure is now used as a matter of course in the surgical treatment of acute pulmonary embolism.
… and today
Surgical pulmonary embolectomy is now performed via median sternotomy. The pericardium is opened and the heart-lung machine (HLM) is connected, as in other cardiac surgical procedures. Either surgery is performed on the beating heart or the heart is cardioplegated and surgery is performed during cardiac arrest. The advantage of beating heart surgery is that no additional ischemic myocardial damage is set if the right ventricle is already compromised.
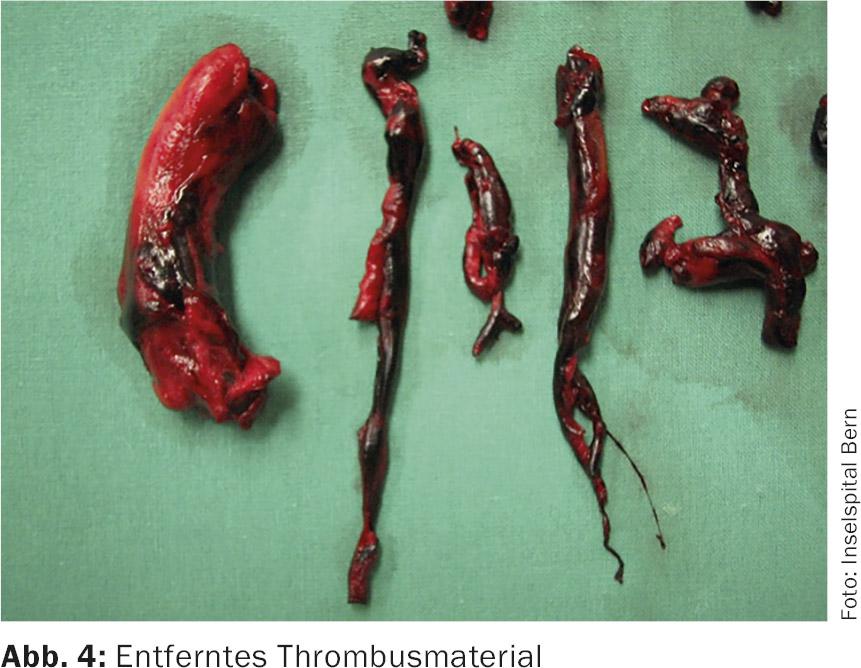
The main pulmonary trunk is opened and the incision is extended into the left pulmonary artery. The thrombus material is removed under visualization (Fig. 4) . The right pulmonary artery is dissected free between the aorta and superior vena cava, opened separately, and the thrombus material is removed in a similar manner. A flexible angioscope is used to verify removal of all thrombotic material as completely as possible. After closure of both pulmonary arteries with continuous suturing, the patient is weaned from HLM. Routine use of intraoperative TEE at the start of surgery looks for patent foramen ovale or intracavitary thrombus – if present, treat accordingly.
The critical moment of surgery is weaning from HLM. The majority of patients referred to surgery are in hemodynamically critical condition. Surgical intervention is often considered a bail-out procedure after unsuccessful fibrinolysis or in patients in severe cardiogenic shock, sometimes with mechanical resuscitation. The right ventricle is often severely compromised in these situations and may ultimately be a limiting factor in the success of surgery. In this situation, temporary extracorporeal membrane oxygenation (arterio-venous ECMO) offers the possibility of giving the right ventricle a few days to recover.
Outcome
Pulmonary embolectomy is a surgically manageable procedure and can be performed with very good results in patients who present for surgery in a hemodynamically stable condition. The correct indication is important. Difficulties may be offered by so-called “acute on chronic” pulmonary emboli. If acute surgery is performed in a patient with chronic recurrent pulmonary emboli, removal of the embolic material from the central and paracentral pulmonary vessels may not significantly improve the already chronic pulmonary arterial hypertension, and the patient does not benefit from surgery. In the acute situation, the more extensive procedure of pulmonary endarterectomy may have to be performed, with a correspondingly higher risk.
According to the current guidelines, surgery for acute pulmonary embolism is recommended in “high-risk” patients in whom systemic thrombolysis is contraindicated or has not been successful [1]. In addition, it can be evaluated in intermediate-risk patients if the risk of bleeding under thrombolysis is considered too high. In practice, this can lead to (too) late referral to surgery. For a long time, therefore, surgical treatment was generally given only to patients who were already in very poor condition (bail-out situation). For this reason, the mortality rates of pulmonary embolectomy were very high. A review of the results showed a mortality rate of more than 30% in the years before 1985 and a mortality rate of 20% in patients operated on after 1985 [6].
In 2008, our group published a series of 25 patients who had undergone surgery between January 2000 and March 2007 [7]. Eighteen patients underwent cardiogenic shock (eight under mechanical resuscitation). The 30-day mortality was only 8% and this despite the poor baseline conditions. Two series with very good results of pulmonary embolectomy were published last year. In the largest series of 115 successive patients, just under half underwent surgery in a “high-risk” situation. This group had a mortality of 10.2% vs. 3.6% in those patients operated on without hemodynamic limitation. However, clear signs of right heart dysfunction were present in both groups [8]. An even lower 30-day mortality of 4.2% overall (and only 1.2% in “intermediate-risk” patients) was found in another series of 96 patients [9].
Currently, a study is starting at our center in cooperation with our colleagues in angiology, in which patients with acute pulmonary embolism in “high-risk” and “intermediate-risk” situations are included and randomized to receive either catheter-based local lysis or open surgical treatment. These data will provide further insight into the differential therapy of acute pulmonary embolism.
Literature:
- The Task Force for the Diagnosis and Management of Acute Pulmonary embolism of the European Society of Cardiology (ESC): 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism. Europen Heart Journal 2014; 35: 3033-3080.
- Kreit JW: The Impact of right ventricular dysfunction on the prognosis and therapy of normotensive patients with pulmonary embolism. Chest 2004; 125(4): 1539-1545.
- Becattini C, Vedovati MC, Agnelli G: Prognostic value of troponins in acute pulmonary embolism: a meta-analysis. Circulation 2007; 116(4): 427-433.
- Meyer G, et al; PEITHO Investigators: Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014 Apr 10; 370(15): 1402-1411.
- Kirschner M: A case of pulmonary artery embolism cured by Trendelenburg’s operation. Archives of Clinical Surgery 1924; 133: 312-359.
- Stein PD, et al: Outcome of pulmonary embolectomy. Am J Cardiol 2007; 99(3): 421-423.
- Kadner A, et al: Excellent outcome after surgical treatment of massive pulmonary embolism in critically ill patients. JTCVS 2008; 136(2): 448-451.
- Neely RC, et al: Surgical Embolectomy for Acute Massive and Submassive Pulmonary Embolism in a Series of 115 Patients. Ann Thorac Surg 2015; 100: 1245-1252.
- Hartmann AR, et al: Acute Surgical Pulmonary Embolectomy: A 9-year Retrospective Analysis. Tex Heart Ins J 2015; 42(1): 25-29.
- Kucher N, et al: Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation 2014; 129: 479-486.
CARDIOVASC 2016; 15(1): 28-32