The success of stroke treatment is largely dependent on the time that elapses between symptom onset and recanalization of the occluded vessel. For this purpose, it is important that symptoms suspicious of stroke are recognized quickly and that immediate referral to a specialized center is made. The standard treatment of acute stroke is systemic thrombolysis with recombinant plasminogen activator (rtPA) within 4.5 hours of symptom onset, although endovascular revascularization is a recommended adjunct to systemic thrombolysis, especially for proximal vessel occlusions.
Stroke is a medical emergency manifested by the onset of an acute focal neurological deficit, such as a sudden onset of speech disturbance, hemiplegia, or visual disturbance. It is among the leading causes of mortality and morbidity worldwide. 85% of all strokes are ischemic and thus the result of a circumscribed circulatory disorder of the brain. Reliable clinical differentiation between these and the remaining 15% caused by intracranial hemorrhage is not possible without instrumental diagnosis. Since the therapeutic concepts of ischemic stroke are fundamentally different from those of hemorrhagic stroke, and the “time is brain” concept essentially applies to the former, we will discuss only ischemic stroke in the remainder of this article.
Penumbra concept
Theory: The concept of “time is brain” in ischemic stroke is largely based on the concept of ischemic penumbra as described, for example, by Astrup et al. 1981 has been described. The ischemic penumbra is defined as a functionally altered but structurally intact brain area that usually surrounds an infarct core. In the ischemic penumbra, a still rudimentary supply of oxygen and nutrients to the cells, e.g. via collateral vessels, is possible, so that cell death is at least delayed. The key thing about ischemic penumbra is that it can transition to a state of permanent and irreversible damage in a relatively short period of time. This time window allows recanalization of an occluded vessel, thus saving the corresponding brain area (penumbra).
In an animal experiment, it was shown that disturbances of neuronal functions occur after regional blood flow falls below a value of approximately 20 ml/100 g/min blood and are reversible after perfusion is regained. If the blood flow continues to decrease or if this state of reduced blood flow persists, irreversible damage to the affected brain area occurs [1]. This ischemic cascade consists of depolarization and washout of toxic amino acids. The influx of calcium and sodium ions results in cytotoxic edema. This activates proteolytic enzymes and releases free radicals, causing further cell damage. This process may spread to the penumbra, resulting in enlargement of the infarct [2].
The “time is brain” concept based on these observations is further supported by estimates that for every minute that a stroke goes untreated, potentially 1.9 million neurons and 14 billion synapses perish [3].
Nowadays, multimodal imaging can be used within minutes to obtain information about the ischemic tissue, i.e., the so-called “ischemic tissue”. infarct core and the penumbra, can be made (Fig. 1a-d, Fig. 2a-d) . This is possible by means of both computed tomography (CT) and magnetic resonance imaging (MRI). Other imaging modalities such as PET and SPECT would also allow this, but are not available quickly enough in the emergency situation.
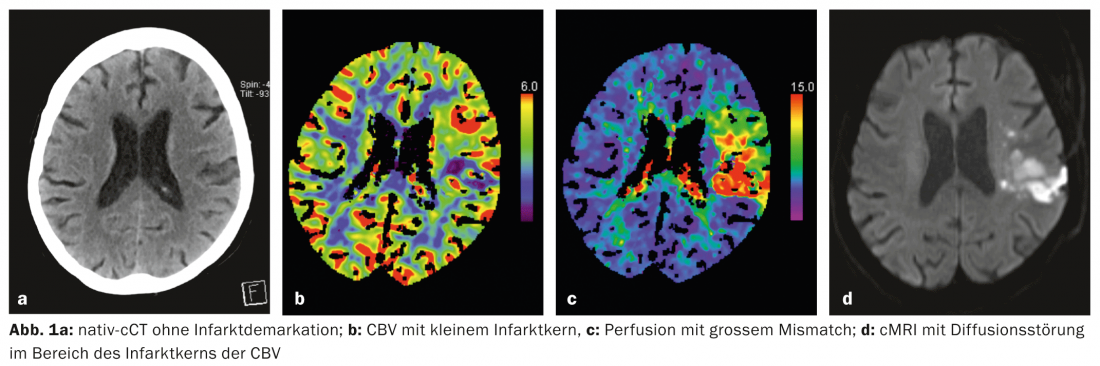
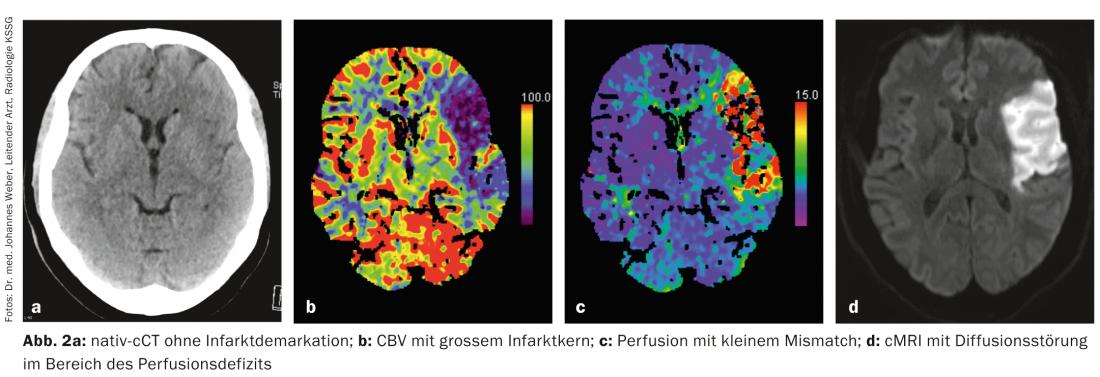
Practice: In stroke medicine, it is important to do everything possible to prevent the expansion of the cerebral infarction in the sense of infarction of the penumbra. An essential component of stroke therapy is recanalization of the occluded vessel as quickly as possible. The only therapy approved for this purpose is systemic thrombolysis with recombinant plasminogen activator (alteplase; rtPA) within 4.5 hours of symptom onset. In selected cases, mechanical thrombectomy by interventional angiography can also be performed.
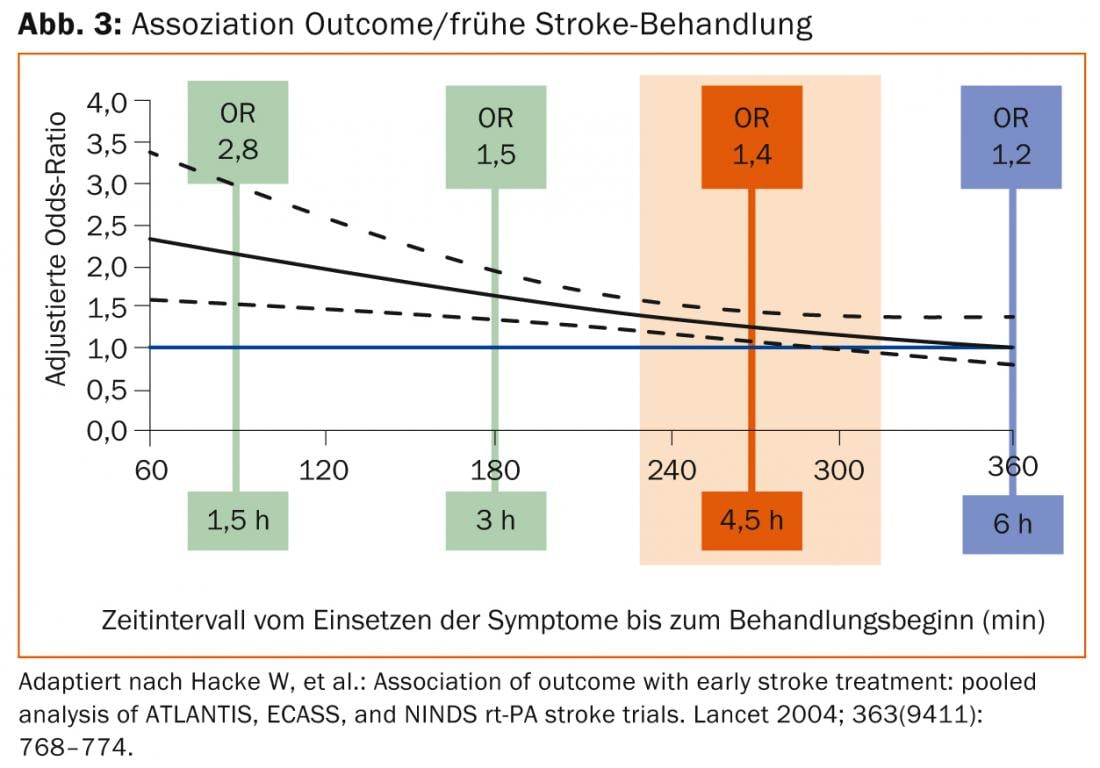
According to the pathophysiology presented, there is a clear time dependence of the success of this therapy. The “number needed to treat” (NNT) for a good outcome is 4.5 if lysis therapy is started within the first 90 minutes. This increases to 9 for onset between 90 and 180 minutes and 14.1 for onset between 180 and 270 minutes (Fig. 3).
Other measures that reduce the spread of infarction include intensive infection prophylaxis and aggressive reduction of elevated body temperatures, optimal control of blood glucose levels, fluid and electrolyte balance, and optimal control of blood pressure and respiration.
Time loss in the treatment pathway of acute stroke
The treatment chain of stroke is divided into the prehospitalization phase (onset of symptoms until hospitalization) and the hospitalization phase (hospitalization until initiation of therapy). Undoubtedly, the most time is lost in the prehospitalization phase [4]. The time loss from the onset of symptoms to the alerting of the emergency medical services depends on the level of knowledge of the population about symptoms suspicious of stroke and about the need to alert the emergency medical services immediately. A next important link is the ambulance service, where recognition of symptoms suspicious for stroke should trigger a predefined referral algorithm with immediate referral to a hospital that can provide stroke acute therapy (stroke units). Direct transfer to a specialized center with the most precise advance information possible leads, as studies have shown, to a faster start of treatment and thus better outcome [5].
Scales such as the “Cincinnati Prehospital Stroke Scale” are used to identify symptoms suspicious of stroke. This scale measures three simple clinical parameters (facial paresis, unilateral arm paresis, aphasia), has a sensitivity of 90% and a specificity of 66% regarding the presence of stroke in the anterior stromal area [6].
Hospitalization phase
Upon arrival at the destination hospital, a rapid diagnosis should be made using basic laboratory (especially blood count and coagulation), vital signs, a symptom-oriented history and standardized clinical examinations (“National Institutes of Health Stroke Scale” [NIHSS]), ECG, and most importantly cerebral imaging. The processes in the target clinic should be structured so efficiently that
- imaging (CT or MRI) can be performed no later than 25 minutes after arrival (“door-to-CT” time).
- systemic thrombolysis can be performed no later than 60 minutes after arrival (“door-to-needle” time).
- the patient can then be further monitored and treated in a stroke unit [7].
Proximal vessel occlusions show low recanalization rates with systemic thrombolysis, so the option of endovascular (catheter) treatment should be evaluated in these cases. In cases of ischemia associated with basilar artery thrombosis, such therapy is even possible up to twelve hours after symptom onset. The decision as to which patients can be assigned to these therapies must be made on a case-by-case basis by experienced stroke specialists.
Time saving in the prehospitalization phase
Public relations: In order to achieve a more rapid referral of stroke patients to a stroke unit, public education campaigns, which must be carried out at regular intervals, have proven effective, as otherwise the effect does not last for more than five months. Experience shows that future campaigns should focus more on specific target groups such as the elderly, minorities, neighbors of stroke survivors, medical students, and even children as potential future patients, relatives, or future physicians [8].
Advance information: Both the German Society of Neurology and international professional societies recommend that the targeted hospital be informed in advance about the arriving patient. This enables early activation of the stroke team and provision of CT, which can significantly reduce time to therapy and increase the rate of thrombolysis.
Time saving in the hospitalization phase
To enable a smooth process and optimal coordination between the disciplines involved, defined action algorithms are indispensable. These include:
- Initiation of a “lysecall” by the neurologist on duty immediately upon receipt of the notification to notify all parties involved
- Have the shock room, stroke team (experienced neurologist and certified nurses), CT, and radiologist on standby.
Telemedicine: In order to enable the fastest possible thrombolysis in a patient for geographically remote hospitals without the corresponding expertise and personnel resources, the concept of telemedical connection to a stroke center with the possibility of live interaction and transmission of findings was developed.
Literature:
- Heiss WD, et al: Ischemic penumbra: evidence from functional imaging in man. J Cereb Blood Flow Metab. 2000; 20(9): 1276-1293.
- Sitzer M, Steinmetz H: Textbook Neurology. Urban&Fischer 2011; 121-122.
- Saver JL, et al: Time is brain – quantified. Stroke 2006; 37: 263-266.
- Puolakka T, et al: Sequential analysis of pretreatment delays in stroke thrombolysis. Acad Emerg Med. 2010; 17(9): 965-969.
- Pe’rez de la Ossa N, et al: Influence of direct admission to Comprehensive Stroke Centers on the outcome of acute stroke patients treated with intravenous thrombolysis. J Neurolog 2009; 256(8): 1270-1276.
- Kothari RU, et al: Cincinnati prehospital stroke scale: reproducibility and validity. Ann Emerg Med 1999; 33: 373-378.
- National Institute of neurological disorders and stroke. Proceedings of a National Symposium on Rapid Identification and Treatment of Acute Stroke 1996.
- Haass A, et al: Time is brain. Neurologist 2013; Nov 27. [Epub ahead of print].
InFo Neurology & Psychiatry 2014; 12(2): 4-6.