In Switzerland, approximately 300,000 labile blood products are transfused per year. Approximately ten adverse events per 1000 transfusions are reported. With increasing knowledge of the individual transfusion reactions, the number of unreported events should continue to decrease and transfusion safety should increase.
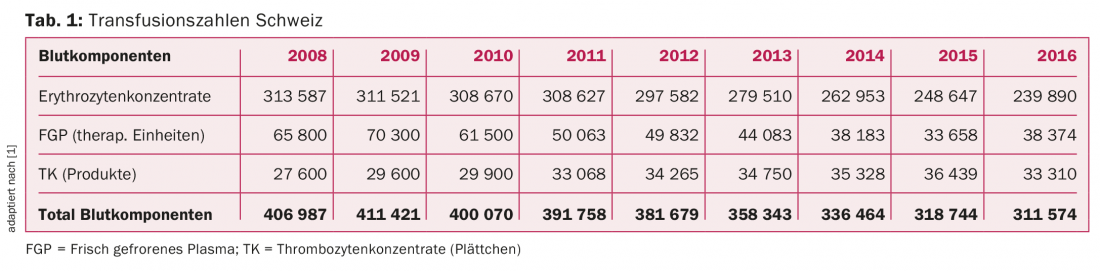
In Switzerland, approximately 300,000 labile blood products are transfused per year. The total number has declined significantly over the last nine years (Table 1). In particular, red cell concentrates (RCC) and fresh-frozen plasma (FGP) are being used much less in hospitals, where “blood patient management” is being increasingly applied, and their use is being critically scrutinized. The number of platelet concentrates (TCs) transfused, on the other hand, shows a continuous increase, which for the first time did not progress in 2016 [1].
The blood products manufactured must meet defined quality standards. Since 1999, all products have been leukocyte-depleted by filtration, and since 2011, TK have been pathogen-inactivated [2].
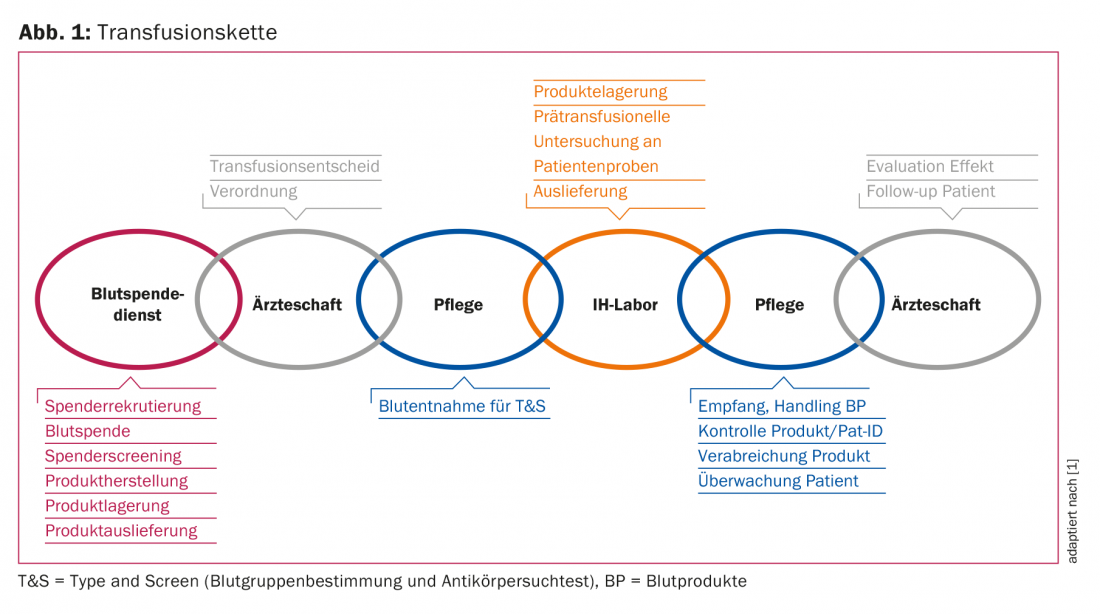
Adverse events associated with transfusion are subject to a systematic surveillance system, the Haemovigilance System [3]. This refers to the entire “transfusion chain” from collection and production, transport, storage, prescription, pre-transfusion testing and administration of the blood products (Fig. 1) . The aim is to identify quality deficiencies or risks at an early stage so that countermeasures can be taken.
The following occurrences in particular are recorded as adverse events:
- Transfusion reactions (TR)
- Incorrect transfusion, incorrect blood product
- “Near-miss” events (near-miss transfusions).
- Donation side effects
- Quality defects and protective measures
According to the Therapeutic Products Act, both users and manufacturers in Switzerland are required to notify the Swiss Agency for Therapeutic Products, Swissmedic [3]. Haemovigilance notifications are submitted to Swissmedic by means of prescribed notification forms (www.swissmedic.ch/haemovigilance-meldungen) by the institutions’ haemovigilance officers. In addition to recording the product, the patient’s symptoms, the severity of the reactions as well as the causal relationship are recorded. In addition, alloimmunizations detected later in the laboratory as well as transmitted infectious diseases are counted as TR.
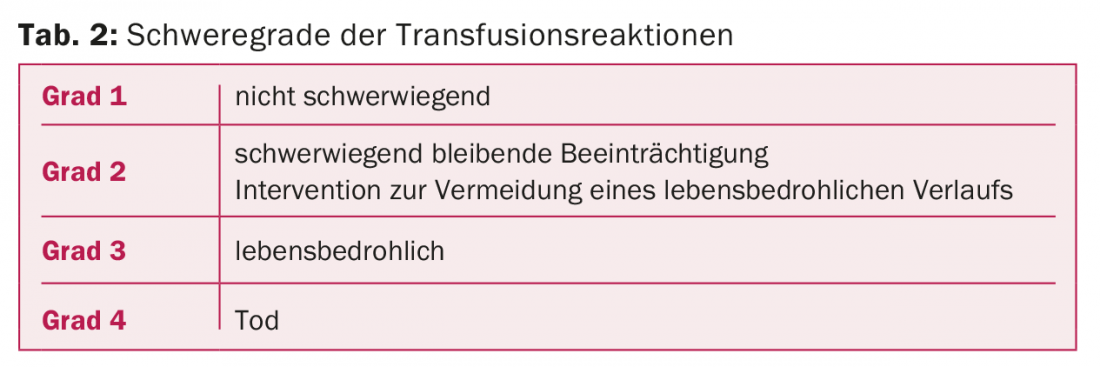
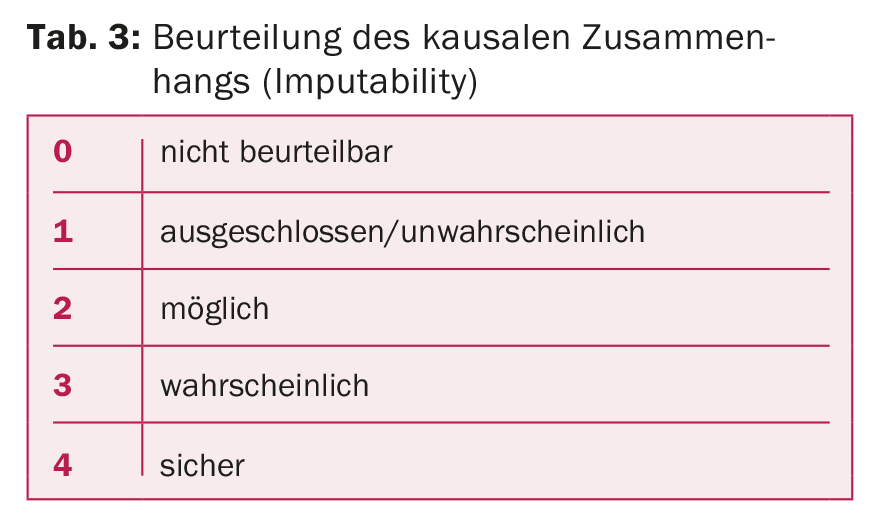
TR are classified into four degrees of severity (Tab. 2). Independently of this, the possible causal relationship between transfusion and the symptoms that have occurred must be assessed, the so-called “imputability” (Tab. 3). Incorrect transfusions and “near-miss” events are classified separately.
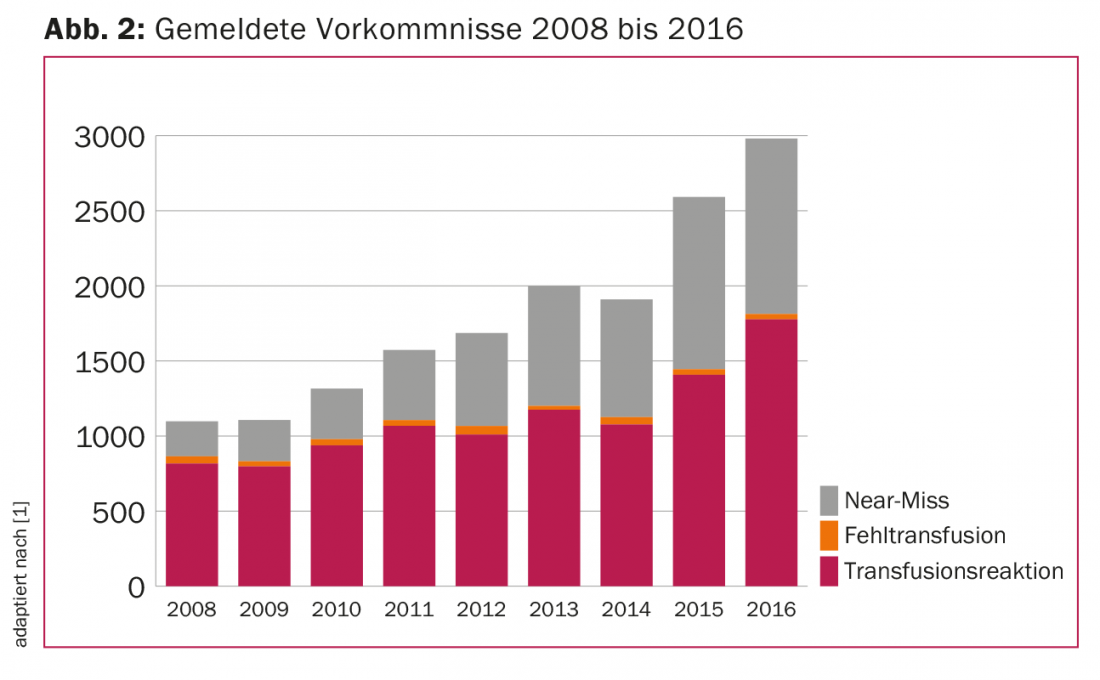
The last nine years showed an increase in reported TR and “near miss” events with the same number of transfusion failures. In 2016, TRs were 1777 and near-miss events were 1168 with 36 transfusion failures (Fig. 2), for a total of 3127 events reported. This corresponds to a reporting rate of approximately ten events per 1000 blood products administered, or 1%. Even though this reporting rate is high by international standards, it can be assumed that there is significant “under-reporting”, i.e. many events are not recorded.
General symptoms
TR show the following symptoms, which may occur singly or in combination during or after a transfusion:
- Unrest
- Heat sensation
- Chills, fever
- Urticaria
- Dyspnea
- Cold sweating
- Thoracic tightness
- Low back/lumbar pain
- Nausea, vomiting
- Hypotension
- Paleness
- Bronchospasm
- Shock
- Increased bleeding intraoperatively
General symptoms may be markedly masked during anesthesia.
Immediate measures
In case of occurrence of above symptoms, the following immediate measures should be taken:
- Interruption of the transfusion
- leave venous access
- Monitor and secure vital functions
- Verify the identity of the patient
- If hemolytic or febrile TR is suspected, collection of native and EDTA blood, citrate blood if necessary.
- Return of the sterilely sealed blood product with transfusion set to the immunohematology laboratory
- Taking blood cultures from the patient
- Antipyretics, antihistamines, steroids
Characteristics of the different forms of TR
Acute hemolytic TR is usually acute intravascular hemolysis due to AB0 incompatible blood. This is triggered by naturally occurring anti-A or anti-B IgM antibodies or complement-activating IgG1 and IgG3 antibodies, which activate complement to the “membrane attack complex” and can thus lyse erythrocytes.
- Symptoms: Chills, fever, nausea, vomiting, hypo/hypertension, tachycardia, sweating, anxiety, “feeling of annihilation,” flank/loin pain, dyspnea, infusion site pain, diarrhea, oliguria, hematuria, bleeding, shock.
- Laboratory: Hb drop >2 g in 24 h, LDH and billirubin rise, haptoglobin drop, free hemoglobin, hematuria.
- Diagnostics: Determination of the blood group from the patient (before and after transfusion) and from the EC, direct anti-globin test (DAT), compatibility test, antibody screening test (AKST) for the detection of alloantibodies, blood count, creatinine, coagulation status, D-dimers.
- Complications: Shock, renal failure, disseminated intravascular coagulation (DIC), multiorgan failure.
- Therapy: volume administration, circulatory support, repeat transfusion
Delayed hemolytic TR is characterized by a hemoglobin drop and billirubin rise 24 h to 28 days after transfusion, occasionally accompanied by a temperature rise mediated by enhanced preexisting or, less commonly, newly emerging alloantibodies to blood group antigens. This leads to a loading of the erythrocytes with immunoglobulins and complement up to complement component C3, so that the erythrocytes are increasingly degraded extravasally by macrophages in the liver and spleen.
- Symptoms: increased temperature, fever, chills, fatigue, jaundice, decreased performance, back pain, (kidney failure).
- Laboratory: Hb drop, LDH and billirubin rise, haptoglobin drop, reticulocytosis, hematuria.
- Diagnostics: direct anti-globin test (DAT), AKST: typically anti-E, anti-Fy (a), anti-Jk (a), often detectable after four days at the earliest
- Complications: rare with further consideration of antibodies.
- Therapy: none as long as asymptomatic, diagnosis and documentation allo-antibodies, transfusion card for patients
Febrile non-hemolytic TR (FNHTR) is characterized by an increase in temperature, usually triggered by leukocytes or cytokines. Since leukocyte depletion of blood products, it occurs much less frequently.
- Symptoms: mild corresponding to temperature rise <2 °C or >38 °C, severe temperature rise >2 °C or >39 °C, myalgias, hypo/hypertension
- Laboratory: CRP, exclusion of hemolysis
- Diagnostics: Blood culture of patient and product
- Complications: Infections
- Therapy: antipyretic therapy, transfusion stop in case of severe reaction
Allergic TR often occurs immediately or in close temporal relation to the transfusion. The allergic reaction, is triggered by plasma proteins, IgE, allergens, very rarely by IgA.
- Symptoms: pruritus, urticaria, dyspnea, bronchospasm, stridor, hypotension, abdominal cramps, diarrhea, chills.
- Laboratory: in case of repeated occurrence, clarification of IgA deficiency in the patient and test for the presence of IgE antibodies against IgA.
- Complications: anaphylactic shock
- Therapy: depending on severity, antihistamines (H1 and H2), steroids, volume, epinephrine, circulatory support, in case of positive evidence of IgA deficiency and relevant antibodies washed products or products from IgA deficient donors.
Transfusion-associated circulatory “overload” (TACO) is triggered by high volume administration or too high a transfusion rate. Their symptomatology starts already during the transfusion or shortly after.
- Symptoms: Dyspnea, cyanosis, orthopnea, cough, tachycardia, more likely hypertension.
- Diagnostics: auscultation, clinical examination
- Complications: Pulmonary edema, especially in heart failure
- Therapy: Stop transfusion, oxygen administration, diuretics, adjustment of transfusion rate
Transfusion-associated acute lung injury (TRALI) is a radiologically confirmed “acute respiratory distress syndrome” (ARDS) caused by anti-granulocytic or anti-HLA antibodies that occurs within 2-6 h after transfusion initiation.
- Symptoms: Dyspnea, cyanosis, hypotension, temperature rise.
- Diagnostics: radiological evidence of infiltrate on both sides, exclusion of hypervolemia, examination of donor for anti-granulocytic or anti-HLA antibodies or antigens in the patient.
- Complications: respiratory failure
- Therapy: oxygenation, circulatory support
To be distinguished from these is isolated transfusion-associated dyspnea, neither TACO nor TRALI, in which dyspnea occurs without evidence of either of the aforementioned characteristics.
Isolated hypotensive TR is also observed, in which there is a systolic blood pressure drop >30 mmHg without allergic symptoms. Most often, symptoms are reversible with interruption of transfusion; rarely, they require minor circulatory support.
In posttransfusion purpura (PTP), a decrease in platelet count occurs approximately one week after transfusion.
Pathogenetic factors postulated include an immune reaction against platelet antigens. Other causes such as immune thrombocytopenia (ITP) or drug interactions should be excluded. Immunoglobulins may be administered in cases of severe bleeding.
Post-transfusion graft-versus-host reaction (GVHD) or “graft versus recipient reaction” results from transfused lymphocytes forming an immune response against the recipient. This involves fever, skin rashes, diarrhea and deterioration of liver function. Immunocompromised patients are at particular risk for this. The reaction can be avoided by irradiating the blood products.
In principle, it is possible for bacterial or viral infections to be transmitted by blood products. The introduction of pathogen inactivation minimizes this for TC stored at 24°C. The blood donation services report positive infection markers, such as for hepatitis B, malaria, etc., to Swissmedic. As methods become more sensitive, donations can be destroyed in a timely manner. If positive results subsequently become known for blood products that have already been used, so-called “look-back” or traceability procedures must be initiated.
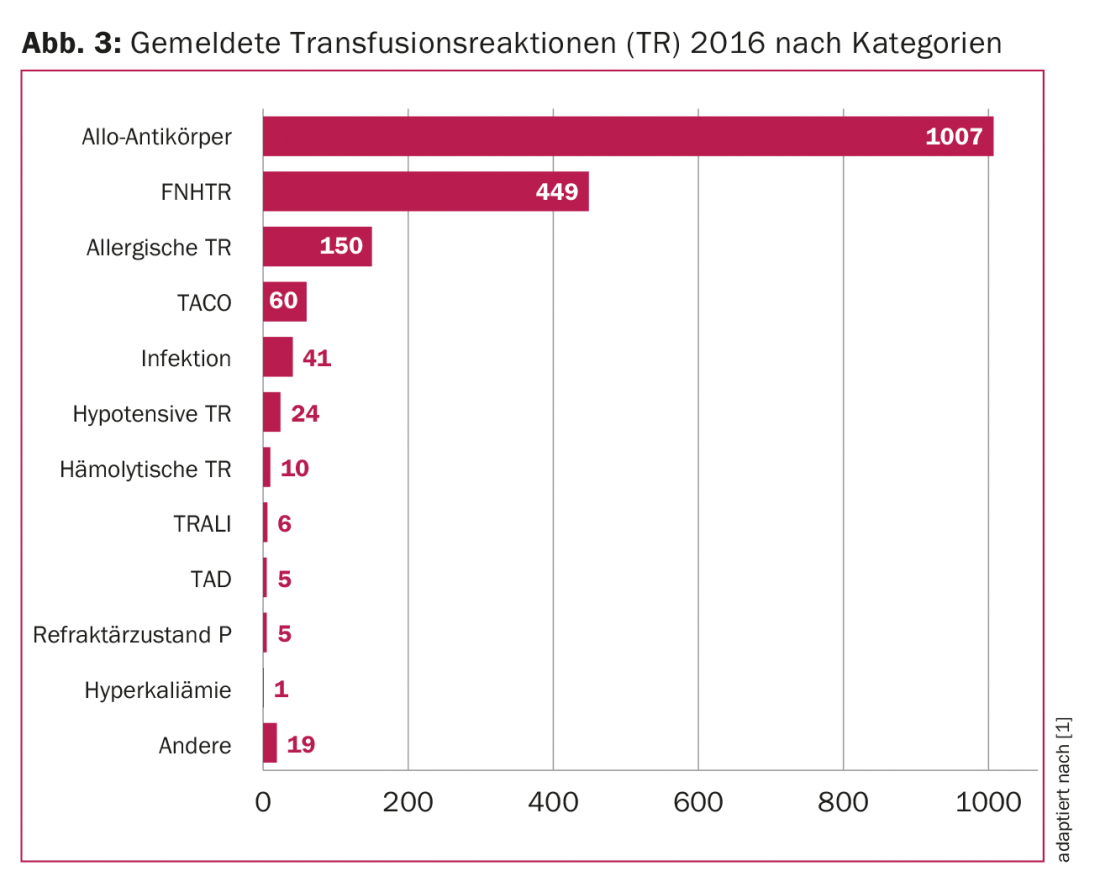
In summary, ten adverse events per 1000 transfusions are currently reported in Switzerland, of which 56% are transfusion reactions with varying frequencies (Fig. 3) . With increasing knowledge of the individual transfusion reactions, the number of unreported events is likely to decrease further.
Take-Home Messages
- In Switzerland, approximately ten adverse events per 1000 transfusions are reported, 56% of which are transfusion reactions (TR).
- In transfusion reactions (TR), characteristic clinical conditions, laboratory findings as well as severity and causal relationship are assessed.
- Increasing “awareness” and consistent application of the Haemovigilance System can continue to increase transfusion safety.
Literature:
- Swissmedic: Haemovigilance Annual Report 2016. (www.swissmedic.ch/swissmedic/de/home/news/mitteilungen/haemovigilance-in-der-schweiz-2016.html)
- Swiss Transfusion SRC, production and specifications of blood products. (https://sbsc-bsd.ch/dokuman2/de-de/bsd/vorschriften/kapitel.aspx)
- The Federal Assembly of the Swiss Confederation: Federal Act on Medicinal Products and Medical Devices (Therapeutic Products Act, TPA), as of 01.01.2018 (www.admin.ch/ch/d/sr/812.21).
CARDIOVASC 2018; 17(1): 24-27











