Despite progress in the understanding of risk factors for aneurysm rupture, the decision whether or not to treat an unruptured intracranial aneurysm (UIA) should be treated remains a challenge. A multidisciplinary team of neurovascular surgeons and interventional radiologists with broad experience in the field should make this decision under critical consideration of their own skills, complication rates and treatment outcomes. Preventive aneurysm occlusion should be proposed whenever the risk of the natural course is estimated higher than the risk associated with the treatment. While the treatment risks have been well documented, a growing body of literature nowadays guides the physician to estimate the risk of aneurysmal subarachnoid hemorrhage from an UIA. Scores like the PHASES score somewhat facilitate the estimation of the rupture risk and prove helpful for the discussion with the patient – whether to treat or whether to follow the aneurysm.
Given the incidence of aneurysmal subarachnoid hemorrhage (aSAH) in Switzerland (7–11/100,000/year) and the high prevalence of unruptured intracranial aneurysm (UIA) of 2%, general physicians are often faced with patients having suffered aSAH or harboring UIA. Every patient with aSAH must be referred to a neurovascular service in order to facilitate prompt emergency care and aneurysm occlusion. Although usually less urgent, patients with newly diagnosed UIA should likewise be counseled by an interdisciplinary team of experienced neurovascular surgeons and interventional neuroradiologists. This review provides current recommendations on the management of UIAs on the basis of the recent literature and latest developments in the understanding of the intracranial aneurysm disease.
aSAH is a life-threatening disease with a case-fatality of about 30% and significant long-term neurological and neurocognitive morbidity in surviving patients [1]. It usually affects young and otherwise healthy people in their productive life years (peak-incidence in the fifth decade) and thus accounts for significant expenses of the health-care system and impact on long-term quality of life (QoL). Warning signs of aSAH are only rarely encountered but include sentinel headache (aneurysm growth), warning leaks (minor hemorrhage), cranial nerve palsy (by compression of an enlarging aneurysm) and micro emboli from partially thrombosed aneurysms. Despite the high prevalence of UIA (about 2% in the general population; 160 000 people in Switzerland), no general screening for UIA is currently recommended [2]. This is justified by the relatively low rate of aneurysm rupture of about 7–10/100,000/year (Switzerland: 560–800 persons/year) with most of the UIA remaining asymptomatic life-long.
The fact that the elective treatment of UIA is likewise associated with 0,5–0,7% mortality and approximately 3–17% morbidity speaks against preventive treatment of all lesions and has to be weighted carefully against the natural course of the disease [3]. Thus, in times of increasing diagnostic cerebral studies and frequently encountered UIA, a challenging task for neurovascular surgeons and radiologists is to appropriately select those patients that will benefit from preventive treatment. This is a difficult challenge that requires excellent knowledge of the disease itself and the current literature. Therefore, we strongly recommend referring every patient with newly diagnosed UIA to an interdisciplinary team of neurovascular surgeons and radiologists for evaluation, follow-up and treatment. Some recently gained insight in the pathophysiology of the disease and the use of established scores (e.g. PHASES) help the clinician in the decision-making today. As the acute treatment of aSAH has been well documented [4], this review will focus on the less frequently described decision-making process of whether or not to treat an UIA.
Types of aneurysms and pathophysiology
There are different types of intracranial aneurysms, whereas the by far most frequent type is the saccular or «berry-like» aneurysm on which this review will concentrate. These lesions are believed to originate from a disregulated vessel wall remodelling induced by turbulent blood flow in combination with vascular risk factors such as elevated blood pressure, smoking or atherosclerosis, usually in the area of a vessel bifurcation (75%) or carotid siphon side-wall (25%). Intracranial arteries are prone to degenerative changes as they have a single internal sheet of elastin and a reduced media and adventitia, as compared to extracranial arteries. There is a genetic predisposition with familial forms, the best known being polycystic kidney disease. Rare entities include mycotic or oncological aneurysms, which are caused by the release of proteases by bacteria or tumor cells digesting the vessel wall. Those aneurysms are treated by antibiotics and chemiotherapy.
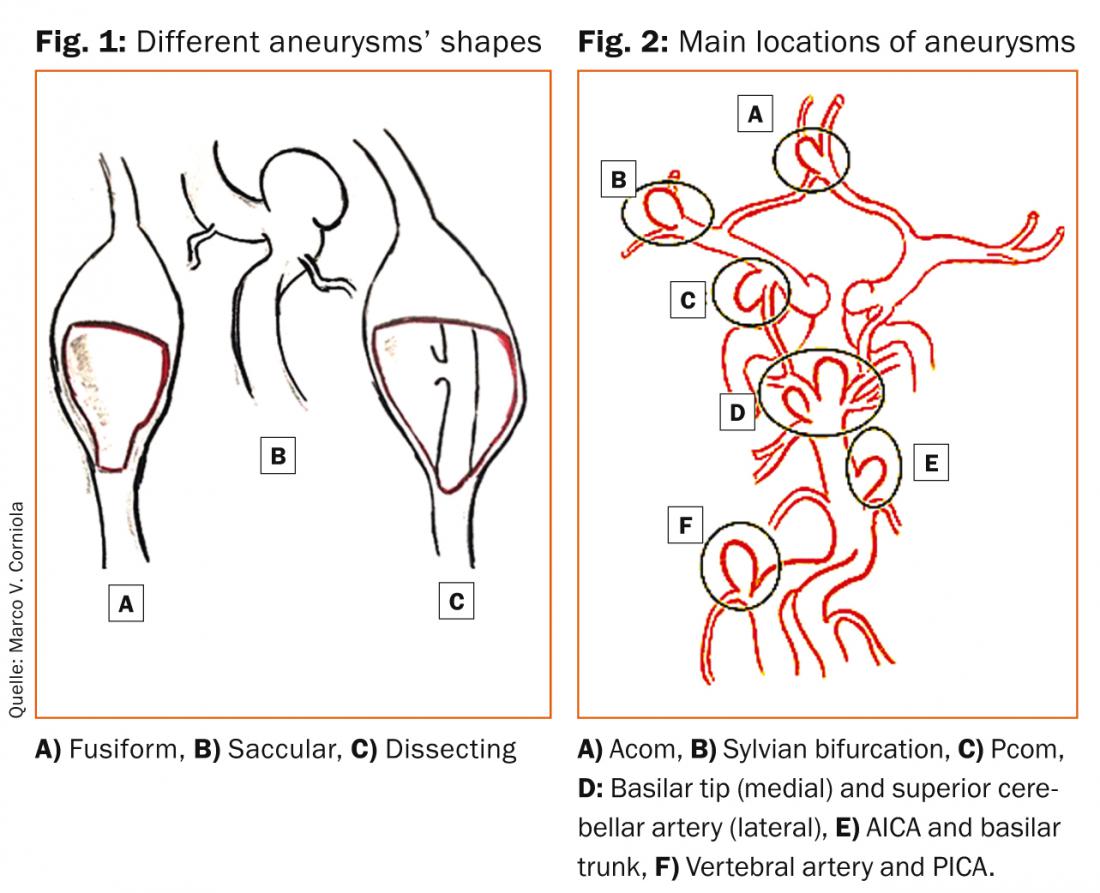
Blood blister-like aneurysms arise from non-branching sites on the supraclinoid internal carotid artery (ICA) and are suspected to originate from an arterial dissection. Likewise, fusiform and dissecting aneurysms originate from an intramural hematoma of the vessel wall initiating the disease, further sustained by local flow alterations. Those aneurysms are most frequently found in the posterior circulation. Figure 1 shows the different shapes of fusiform, dissecting and saccular aneurysms.
Location of aneurysms
About 85% of intracranial aneurysms are located in the anterior circulation, predominantly on the circle of Willis. Common sites include the junction of the anterior communicating artery (AcoA) with the anterior cerebral artery (ACA), the junction of the posterior communicating artery (PcoA) with the internal carotid artery (ICA), and the bifurcation of the middle cerebral artery (MCA). Posterior circulation sites often include the top of the basilar artery (BA), the junction of the BA and the superior or anterior inferior cerebellar arteries (SCA and AICA), the junction of the vertebral artery (VA) and the posterior inferior cerebellar artery (PICA). Figure 2 shows major aneurysms sites on the circle of Willis and posterior circulation.There are several rules mainly formulated by Rhoton and Lasjaunias in 1979 that are helpful to understand the concept of aneurysm location and formation [5]:
- Saccular aneurysms usually arise at branching sites of arteries, including the origin of side branches (e.g. Pcom from ICA), or the subdivision of a main arterial trunk (e.g. MCA bifurcation or BA).
- Saccular aneurysms usually arise at the convex, not at the concave side of a turn or curve of the parent artery, as by local alterations in the intravascular hemodynamics the stress and the force of the pulse wave is most pronounced in these locations.
- Saccular aneurysms point into the direction that the blood would have gone if the curve at the aneurysm site were not present. The aneurysm dome thus points in the direction of the maximal hemodynamic thrust.
- All aneurysms induced by flow increased upstream of an arteriovenous malformation (AVM) regress once the AVM occluded or resected. This principle was not formulated by Rhoton (as the three firsts) but is assumed as a major rule in neurosurgery [6].
Non-hemorrhagic presentation
UIA are a dynamic pathology that may manifest clinically by sentinel headache (warning leak) or by cranial nerve palsy. The risk for aSAH is significantly higher for symptomatic aneurysms, irrespective of their size. Sentinel headache is characterized as sudden, intense, and persistent/recurrent. It usually precedes aSAH by days or weeks as it corresponds to a minor hemorrhage from the aneurysm or be related to aneurysm growth with formation of a fragile bleb on the aneurysm dome. Cranial nerve palsy appears as a result of mass effect of a growing aneurysm. Some of the typical presentations include oculomotor palsy by an aneurysm of the PcoA, abducens palsy by an intracavernous ICA aneurysm, trigeminal palsy by vertebrobasilar aneurysms or aneurysms of the ophthalmic artery causing visual field defects. These symptoms, however, result in the correct diagnosis in only 10–15% of cases before aSAH.
Diagnosis of UIA
Today, most UIA are found incidentally in magnetic resonance imaging (MRI)-studies performed for unrelated complaints. A time-of-flight (TOF) MR-angiography (MRA) does not involve gadolinium contrast agent. Sensitivity and specificity of MRA are similar to those of CT-angiography when interpreted by an experienced radiologist [7], but may be subject to flow artefacts as CTA sensitivity may be affected by the close proximity of aneurysms to skull base and therefore hidden by bone structures. Any UIA identified by MR-A or CT-A that is considered potentially dangerous by a neurovascular specialist, and where treatment may be considered, should be carefully investigated by a digital subtraction angiography (DSA) and 3D-DSA to optimally choose the best treatment strategy.
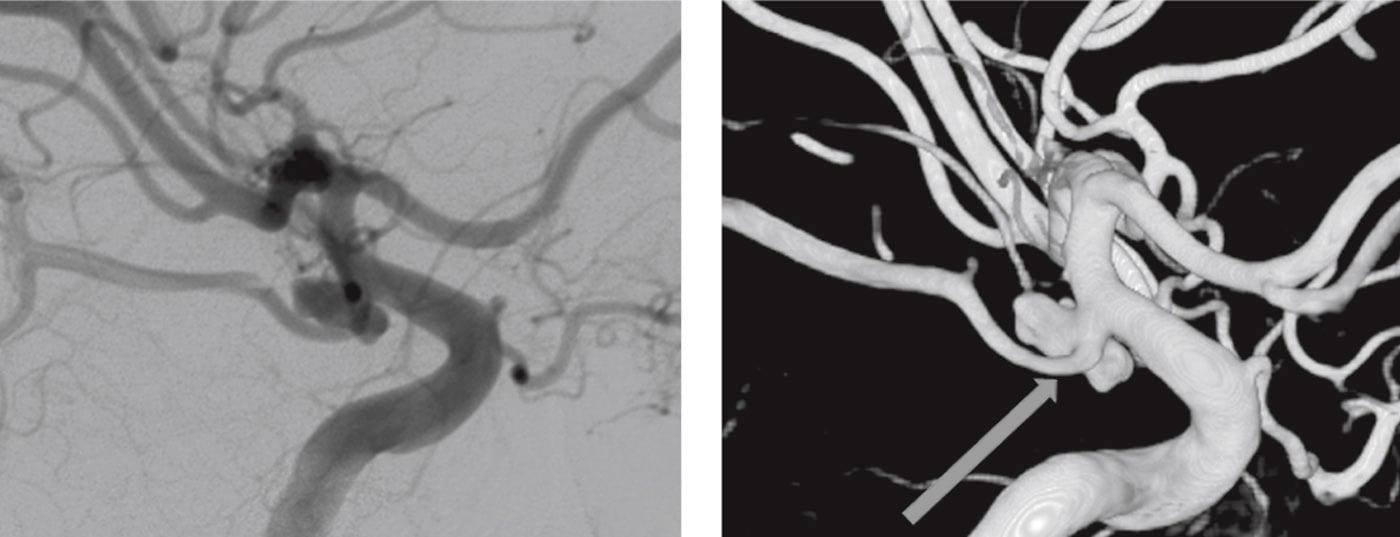
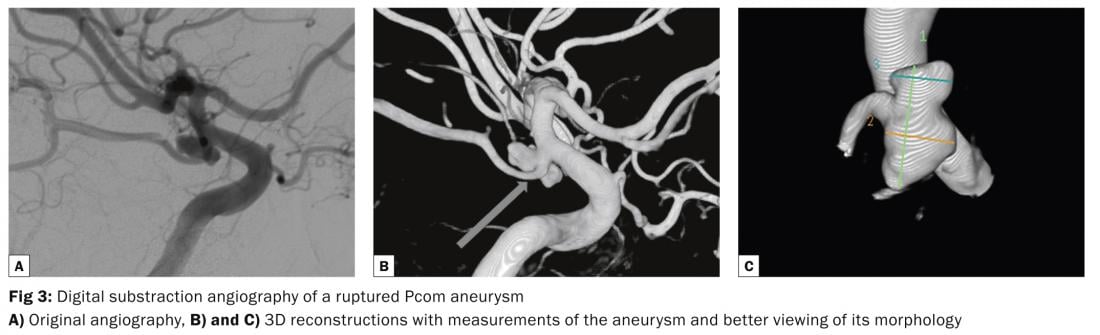
Despite the advances in MR-imaging, the DSA remains the diagnostic gold standard for the diagnosis of intracranial aneurysms (ruptured or unruptured). It allows detailed visualization of the morphology – neck, dome, lobules, «baby aneurysms» or blebs as well as the morphology of the blood inflow jet and impact on the dome – that help to better estimate the rupture risk of UIA (Figure 3). Moreover, the DSA helps to select the treatment modality and estimate the treatment risk by identifying perforating arteries from the aneurysm and determine the collateral circulation. As it is an invasive diagnostic procedure with a risk of neurological complication of about 0,1% the DSA should be reserved for those patients with UIA that are considered potentially dangerous by experts.
Predictors of aneurysm rupture
Clinical risk factors: The decision to treat an UIA should be made if the risk of the natural course (aneurysm rupture with its associated mortality and morbidity) is considered higher as the therapy-related risks. For patients with an estimated high risk of aneurysm rupture, the threshold for preventive treatment should be low and vice-versa. Many factors such as general life expectancy, patient-, aneurysm-, and treatment-specific parameters have to be considered. Known risk factors for aneurysm rupture include unruptured symptomatic aneurysms, aneurysms >10 mm, location in the posterior circulation, positive family history, smoking, female sex, alcohol consumption of >150 g/week, hypertension and presence of multiple aneurysms (table 1) [2,8,9].
The International Study of Unruptured Intracranial Aneurysms (ISUIA) together with the Unruptured Cerebral Aneurysm Study (UCAS) generally suggest to treat UIA that are >7 mm in size if situated in low risk locations (ICA and MCA) [10]. Lesions less than 4 mm are to be treated if situated in high risk regions (mainly posterior circulation) or in patients with any or multiple of the risk factors listed in table 1.
Aneurysm morphology and flow-patterns: With technical progress allowing the computational 3-dimensional analysis of aneurysm and parent vessel anatomy as well as fluid dynamics, the predictive capacity of these factors for aneurysm rupture have been studied and enabled exciting pathophysiological insights. The combination of structural defects of the artery with hemodynamic factors, such as wall shear stress (WSS), pressure, residence time and flow impingement, are thought to play a significant role in the pathogenesis of aneurysm formation and rupture [11]. Hemodynamic factors contribute to thrombus formation in the aneurysm, inflammatory processes of the aneurysm wall and its rupture. Therefore, morphological features are increasingly used to estimate the rupture risk of intracranial aneurysms.
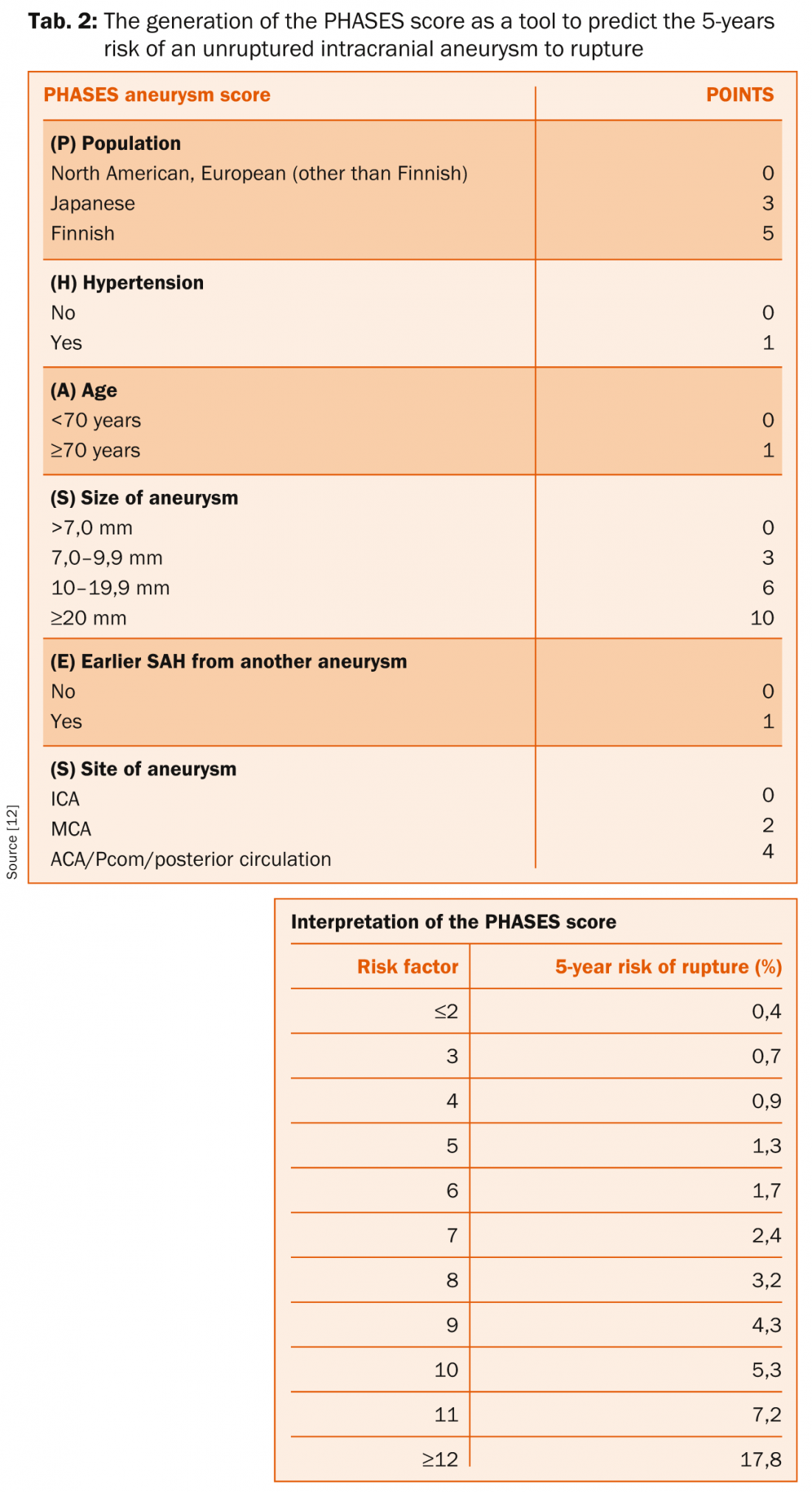
The PHASES-Score: The PHASES-score has been developed to simplify the decision-making whether or not an UIA should be treated. Based on the data of six clinical trials including 8382 patients with 10 272 UIA, the score uses known risk factors for aneurysm rupture (PHASES = Population, Hypertension, Age, Size of the aneurysm, Earlier SAH from another aneurysm, Site of aneurysm) that are available for every patient to estimates the 5-years risk for aSAH (table 2) [12]. While the idea behind this scoring system is to be commended, it has to be emphasized that this score has never been validated in a prospective study and must not necessarily apply to the individual patient. It is of note, that if the life-expectancy is considerably higher than five years, the life-time risk will be much higher than predicted by the score.
Management of UIA and outcome of elective aneurysm treatment
In most cases, saccular aneurysms are excluded from the circulation by either neurosurgical clipping or endovascular treatment. The surgical clipping requires a craniotomy, the preparation of the cerebrovascular anatomy and the placement of a clip on the aneurysm neck. Despite ongoing refinements of the operative techniques, a mortality of about 0,7% and a morbidity rate of 3–17% have to be considered. Endovascular treatment usually comprises the occlusion of the lesion by application of coils directly in the aneurysm sac, with or without additional techniques like balloon-remodeling or the position of a stent in the parent vessel. While the mortality for endovascular treatment of UIA is similar (0,5%), morbidity has been reported to be lower [3]. Which treatment modality is superior in which clinical constellation has been reported recently [4]. However, case selection bias makes it difficult to compare both techniques, but it is generally accepted that both techniques are complementary and that the best results are obtained when the most straightforward approach with the least complexity is chosen by a collaborative multidisciplinary team.
References:
- Stienen MN, et al.: Acta Neurochir (Wien) 2013; 155(11): 2045–2051.
- Rinkel GJ, et al.: Stroke 1998; 29(1): 251–256.
- McDonald JS, et al.: Stroke 2013; 44(4): 988–994.
- Seule MA, et al.: Anasthesiol Intensivmed Notfallmed Schmerzther 2010; 45(1): 8–17.
- Rhoton AL Jr, et al.: Clin Neurosurg 1979; 26: 248–306.
- Meisel HJ, et al.: Neurosurgery 2000; 46(4): 793–800.
- Li MH, et al.: Stroke 2009; 40(9): 3127–3129.
- Bijlenga P, et al.: Stroke 2013; 44(11): 3018–3026.
- Clarke M: Neuroradiology 2008; 50(8): 653–664.
- Wiebers DO, et al.: Lancet 2003; 362: 103–110.
- Xiang J, et al.: Stroke 2011; 42(1): 144–152.
- Greving JP, et al.: Lancet Neurol 2014; 13(1): 59–66.
More information about aneurysmal pathology is available at the SWISS SOS registry (Swiss study on aneurysmal subarachnoid haemorrhage, www.swiss–sos.ch), the swiss nationwide registry of subarachnoid hemorrhage, and:
- Schatlo B, et al.: Introducing a nationwide registry: the Swiss SOS study on aneurysmal subarachnoid haemorrhage (Swiss SOS). Acta Neurochir (Wien) 2012; 154(12): 2173–2178; discussion 2178.
InFo NEUROLOGIE & PSYCHIATRIE 2015; 13(2): 4–8