A number of studies have shown that ultrasound can detect carcinomas in addition to mammography, particularly in the dense breast. In the early detection of cancer in asymptomatic women, breast ultrasonography currently has no recognized independent value. In women undergoing intensified screening, ultrasound is also an integrative part of the multimodal examination program. If a bioptic clarification is indicated, it is preferably performed sonographically guided.
Ultrasound is known to be a practical, easily accessible examination method with good, albeit examiner-dependent, sensitivity. In breast carcinoma diagnostics, a distinction must be made between ultrasound use in the context of early detection and use in cases of suspected breast carcinoma or already confirmed carcinoma.
Technical aspects
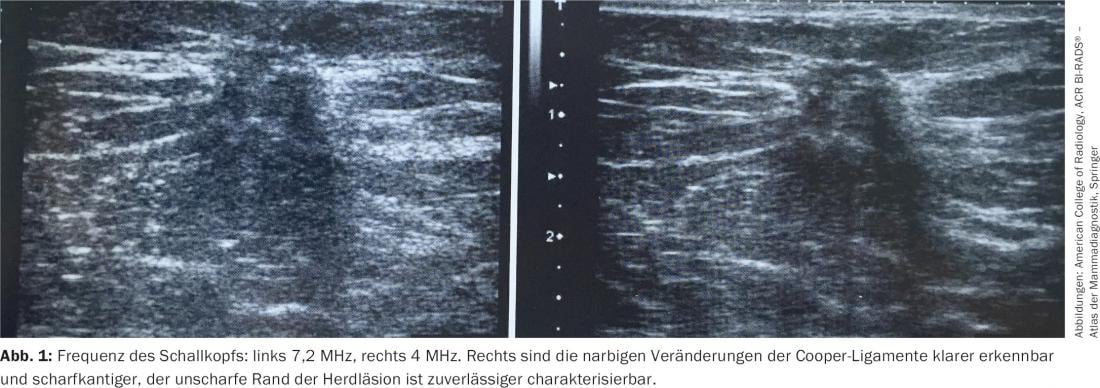
The primary requirement of a breast ultrasound is to provide a high-contrast, high-resolution image with good anatomic representation. Poor ultrasound image quality can lead to serious misdiagnosis, such as mistaking a carcinoma for a cyst. The ACR Practice Guideline for the Performance of a Breast Ultrasound Examination (2011) recommends the use of a wide-bandwidth linear transducer with a central frequency of at least 10 MHz [1]. At the upper end of the high-frequency scale (between 12 and 18 MHz), these transducers provide high-resolution images (Fig. 1) . In the range of lower frequencies, a penetration depth into the tissue of up to 5 cm can be achieved.
The focusing zone should be set in the region of interest in the anterior to middle third between the skin and the thoracic wall. When assessing a lesion, the focal zone is optimally placed in the center of the lesion.
The oldest systematic examination technique is the transverse and longitudinal meandering tracing of the chest. With the increase in spatial resolution in mammary sonograms, the duct system has now offered itself as a guide structure, so that anti-radiographic transducer guidance in segmental transverse and longitudinal sections is becoming more common as an alternative.
Techniques such as Doppler, 3D and 4D analyses, and elastography increase the amount of diagnostic information, but have not yet been defined as necessary for basic diagnostics. The latest technology in this field is automated breast ultrasonography, which achieves user-independent and rapid full-field breast ultrasound examinations.
Ultrasound diagnostics for early detection
Mammography is the only screening method for breast cancer that has been shown to have a positive effect on reducing breast cancer-specific mortality in various population-based studies [2]. Even in times of state-of-the-art therapy concepts, tumor size and especially nodal status at diagnosis remains an essential and undisputed prognostic parameter [3]. This means that the role of imaging in the early detection of breast carcinoma remains central. Of eleven randomized mammography trials, only those that also demonstrated a decrease in advanced breast cancer cases and an increase in node-negative breast cancers showed a reduction in breast cancer mortality [4,5].
In women with dense breast tissue, breast carcinoma may be masked and consequently missed on mammography; this results in an excess of advanced, newly diagnosed breast carcinomas [6]. After a normal screening mammogram, women with extremely high breast density are almost 18 times more likely to be diagnosed with breast cancer based on clinical symptoms than women with involved glandular tissue [7]. These interval cancers (i.e., those diagnosed before the next screening exam) tend to be larger, more aggressive, and have a worse prognosis than cancers detected at screening. Consequently, a low rate of interval carcinoma is a measure of the effectiveness of screening.
Mammographically high breast density plays a role not only in terms of masking breast cancer, but increases breast cancer risk per se. Meta-analyses have demonstrated a four- to fivefold increased risk of developing breast cancer when women with very low breast density were compared with women who had very high breast density [8].
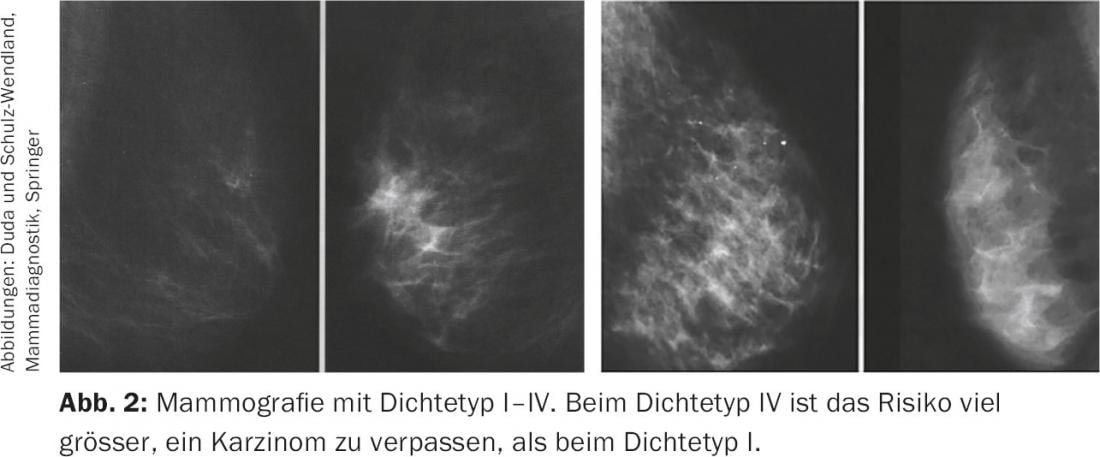
A number of studies have shown that ultrasound can detect carcinomas in addition to mammography, particularly in the dense breast. (Fig.2). The S3 guideline for breast carcinoma therefore already calls for the regular use of breast ultrasonography in mammographically dense breasts (ACR density 3.4) in the early detection guideline from 2008 [9]. Ultrasound is also recommended as a complementary measure for dense breasts in the latest guideline recommendation of the Working Group on Gynecologic Oncology [10]. Because breast density has such a significant impact on the certainty of excluding breast cancer, a law was even passed in the U.S. in 2011 requiring facilities that perform mammograms to provide written information about individual breast density to both the referring physician and the patient.
In the early detection of cancer in asymptomatic women, breast ultrasonography currently has no recognized independent value. Its role in this regard is rather to complement mammography, with the quality of mammography and sonography influencing each other.
An international team of researchers wanted to know if sonography was also suitable for breast cancer screening [11]. For this purpose, approximately 2700 women were examined three times at intervals of one year each by means of mammography and ultrasound. A total of 111 confirmed breast cancer cases were detected. Detection rates were comparable for both assay methods. To detect a case of breast cancer, 129 women had to be sonographed. On mammography, this value was 127. However, the success of ultrasound seemed better with increased breast density. Also, more invasive and lymph node-negative tumors were detected sonographically. The researchers assess sonography as an alternative in countries where structured mammography screening is not offered.
It should not be forgotten that the increased cancer detection rate due to additive breast ultrasonography is offset by an increased biopsy rate. Fortunately, the interventional clarification of an unclear sonographic finding, which can be performed without delay, allows a significantly reduced waiting time compared to alternative methods (stereotactic, tomosynthesis- or even MRI-guided clarification).
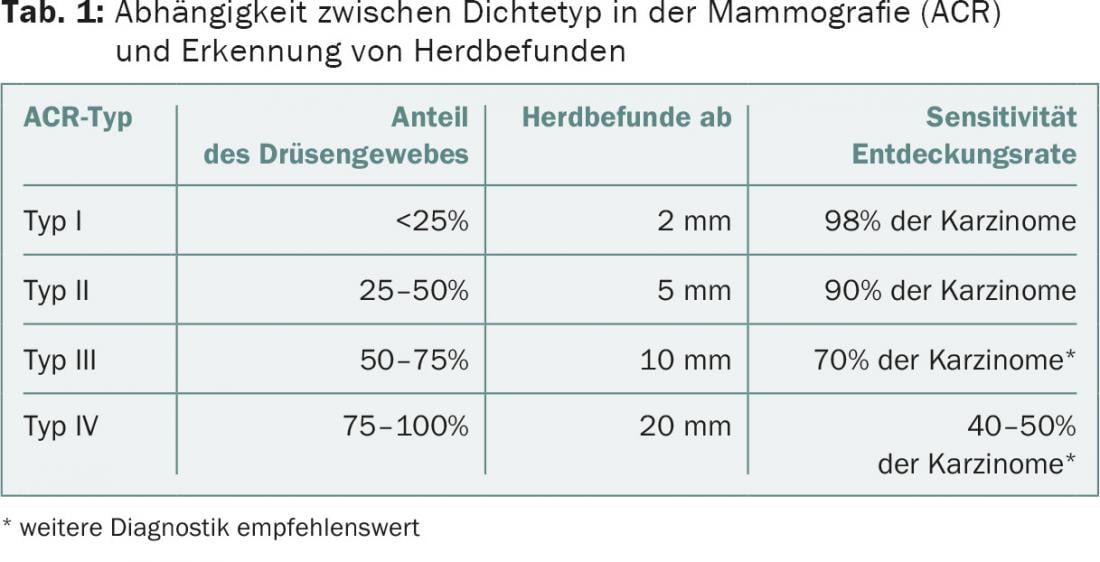
In summary, breast ultrasonography is obligatory in the early detection situation as an additive method to mammography in dense breasts (ACR density 3.4) (Tab. 1) . This also means that breast ultrasonography plays a more important role in the younger woman (with physiologically dense glandular tissue) than in the older patient. Sonographic findings have a higher value in younger women than in older ones and may even surpass mammography in women under 40 years of age in terms of positive malignancy diagnoses.
In women subject to intensified screening (BRCA1 or BRCA2 gene mutation carriers, heterozygote risk ≥20% or residual lifetime risk ≥30% according to standardized prediction model, and survivors after childhood tumors with therapeutic radiatio of the chest wall), ultrasound is also an integrative part of the multimodal examination program (recommended every six months from 25 years of age).
Ultrasound diagnostics in the symptomatic patient
Because breast ultrasonography is a practical and readily accessible method of examination, it is often used in the symptomatic patient early in the diagnostic process. Ultrasound complements other imaging modalities; it allows differentiation between cystic and solid findings. Even if the mammographic correlate of a palpation finding is already presentable, it is important to characterize the mass sonographically, document the tumor extent, and exclude multifocality/centricity and axillary involvement. In patients with abnormal findings on breast MRI and initially unremarkable breast ultrasonography, a target ultrasound should definitely be performed. If the suspicious finding described on MRI can then also be visualized sonographically, a bioptic workup can be performed sonographically rather than MRI-guided. Interventional clarification of an unclear sonographic finding can be performed without delay and significantly shortens the waiting time compared to alternative methods. This means less psychological stress for the patient.
Thus, it is understandable that a bioptic clarification is preferably performed sonographically-guided, provided that the findings can be well visualized on ultrasound. A particularly good indication is the presence of multiple palpable nodes in suspected carcinoma. Ultrasound also comes first in the diagnostic cascade of the breast during pregnancy and lactation. In contrast to stereotaxy, tomosynthesis- or MRI-guided biopsy, sonography-guided biopsy also causes less personnel, equipment, and organizational effort.
Breast ultrasonography is also used to monitor tumor response during neoadjuvant chemotherapy. In these patients, it is usually indicated to mark the tumor with a clip during neoadjuvant chemotherapy so that the site to be operated on can still be traced if there is a complete tumor response at the end of chemotherapy. The insertion of the clip can usually take place sonographically-guided without any problems. Ultrasound is also used to monitor tumor size during primary endocrine therapy of a previously unoperated breast carcinoma (e.g., in an elderly patient or when the recommended surgery is declined). In addition, ultrasound in the preoperative setting helps to delineate the exact incision and to perform wire marking of the tumor if the carcinoma is not palpable but can be visualized sonographically.
Literature:
- American College of Radiology: ACR practice guideline for the performance breast ultrasound examination.
- Broeders M, et al: The impact of mammographic screening on breast cancer mortality in Europe: a review of abservational studies. J Med Screen 2012; 19: 14-25.
- Saadatmand S, et al: Influence of tumour stage at breast cancer detection on survival in modern times: Population based study in 173797 patients. BMJ 2015; 351: h4901.
- Smith RA, et al: The randomized trials of breast cancer screening: what have we learned? Radiol Clin North Am 2004; 42: 793-806.
- Tabár L, et al: Insights from the breast cancer screening trials: how screening affects the natural history of breast cancer and implications for evaluating service screening programs. Breast J 2015; 21:13-20.
- Gierach GL, et al: Relationship between mammographic density and breast cancer death in the Breast Cancer Surveillance Consortium. J Natl Cancer Inst 2012; 104: 1218-1227.
- Boyd NF, et al: Mammographic density and breast cancer risk: current understanding and future prospects. Breast Cancer Res 2011; 13: 223.
- Sickles EA: The use of breast imaging to screen women at high risk for cancer. Radiol Clin North Am 2010; 48: 859-878.
- Albert US (editor): Level 3 Guideline Breast Cancer Early Detection in Germany. 1st update 2008. Germering/Munich: W. Zuckschwerdt Verlag GmbH, Industriestrasse 1, D-82110ISBN 978-3-88603-931-9.
- Schreer I, Albert US: Early detection and diagnosis. In: AGO Breast Committee. Diagnosis and Treatement of Patients with Primary and Metastatic Breast Cancer. Recommendations 2015. www.ago-online.de.
- Berg WA, et al: Current Status of Supplemental Screening in Dense Breasts. JNCI J Natl Cancer Inst 2016; online first.
InFo ONCOLOGY & HEMATOLOGY 2016; 4(6): 11-15.
HAUSARZT PRAXIS 2017; 12(2): 26-30