Iron deficiency anemia is common – especially in elderly patients with renal insufficiency or malnutrition. As a rule, a multifactorial process can be assumed. Therefore, a number of different causes may also come into consideration, which must be considered differentially. When is a hematological workup useful?
When considering laboratory values, it is always important to keep in mind how the reference range is defined. Usually, the mean value of a normal distribution is taken as a basis and two standard deviations are integrated downward and upward. In this way, 95% of the healthy population is included in the reference range, explained Cyrill Rütsche, MD, Basel. The WHO recommends age-adjusted hemoglobin (Hb) levels for the diagnosis of anemia (Table 1) [1]. The living environment of the patients should not be disregarded. This is because differences in altitude also affect hemoglobin concentration. In the case of smokers, 3 g/l should always be deducted from the measured laboratory values, as the values are also influenced here. The higher the nicotine consumption, the more should be credited.
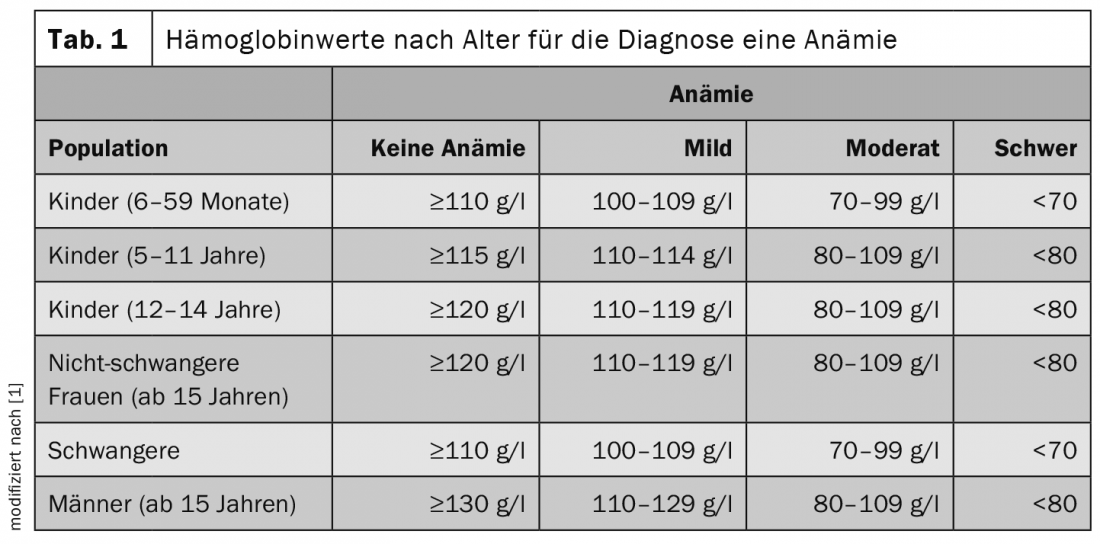
An exciting aspect that has not yet been fully elucidated concerns population-specific thresholds. In America, studies have shown that in the African-American population, hemoglobin is 6-10 g/l lower. Partially, this is explained by a higher proportion of heterozygous alpha-thalassemia. However, this fact is not yet fully understood, according to the expert [2,3]. Other countries could also see a deviation from the 12 g/dL Hb valid in the USA. Therefore, the question is whether a population-specific adjustment needs to be made.
The prevalence of anemia is highest in children and adolescents, decreases in young adulthood, and then slowly increases throughout life [4]. Women are affected more frequently than men. In Western Europe, diabetic and hypertensive kidney diseases were the main causes of iron deficiency anemia [4].
Diagnosis within the framework of an algorithm
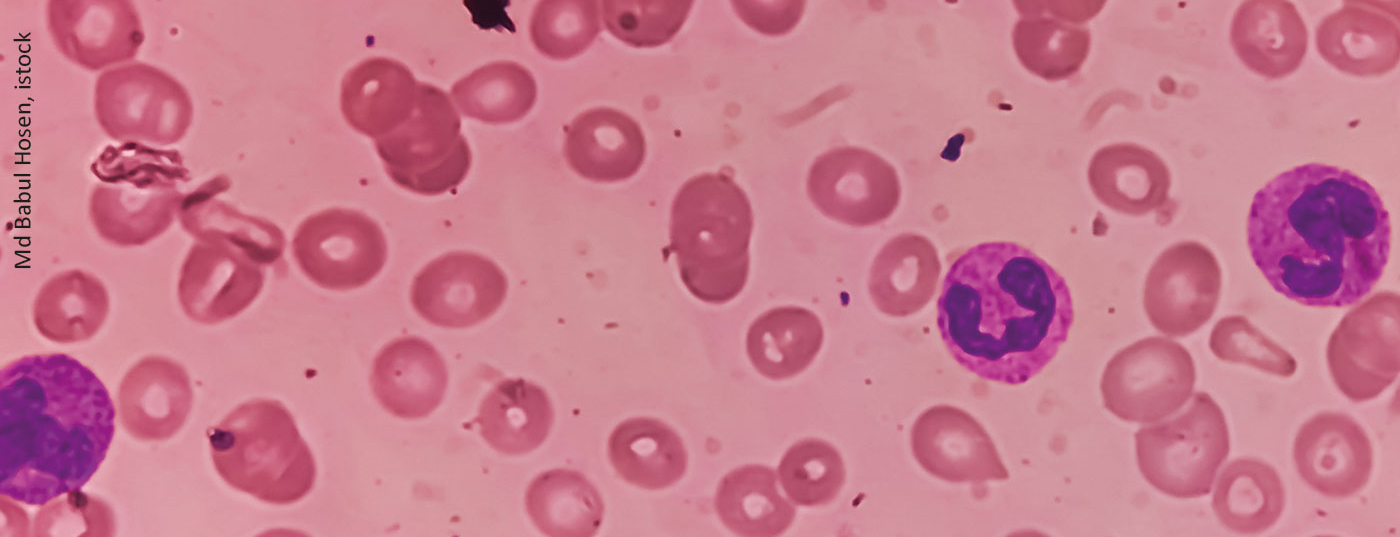
If anemia is suspected, the first step should be to look at reticulocyte values. If these are above 100 G/l, hyperregenerative anemia is the underlying condition, while values <100 G/l are hyporegenerative anemia [5]. In hyperregenerative anemia, the second step should be to look for signs of hemolysis. If bilirubin and/or LDH are elevated or haptoglobin is decreased, a blood smear should be obtained. If fragmentocytes can be detected in this, microangiopathy should be considered. If not, the DAT test will help. If the test is positive, the diagnosis is autoimmune hematolytic anemia including Cold agglutinin disease close. A negative result is more likely to be due to membrane defects, enzymopathies, or hemoglobinopathies. If no signs of hemolysis can be observed, the clarification of blood loss is the primary differential diagnosis.
In the setting of hyporegenerative anemia, antecedent Hb and hematocrit (Hct) levels should be considered. If these have been outside the norm for a long time, hemoglobinopathy or congenital anemia may be present. If normal Hb and Hct values were measured in the previous period, the presence of macrocytosis should be clarified. In case of elevated values, a vitamin B12 and/or folic acid deficiency or a drug-related cause should be considered. If one can be detected, megaloblastic anemia should be considered. If not, diagnoses such as hypothyroidism, MDS, plasma cell myeloma, or congenital anemia are more likely. If there is no macrocytosis but microcytosis, the next step is to evaluate iron deficiency. If this can be confirmed, blood loss should be investigated. If not, it could go into areas of inflammation or thalassemia. If microcytosis can also be excluded, diagnoses such as hypothyroidism, renal insufficiency, inflammation, or PRCA may be considered.
Hemoglobinopathy in focus
If hemoglobinopathy is suspected, it is useful to look at the patient’s home. A person from Scandinavia, for example, is unlikely to have such a condition. For patients from Central Africa or Southern Italy, however, the situation is different. In Southeast Asian countries, there is also an increased incidence of HbE, Rütsche reported. If suspected, Hb chromatography and a safety cell test should be performed. However, the exclusion of alpha-thalassemia is only possible by molecular genetics [6].
The anemia of chronic diseases
Iron metabolism is controlled by hepcidin, which is synthesized in the liver. Hepcidin inhibits both iron uptake and iron release. However, cytokines – most notably IL-6 – lead to increased hepcidin synthesis and concomitant reduced erythrocyte lifespan. In renal failure, this is further compounded by the fact that hepcidin clearance is decreased. Accordingly, iron deficiency is common in patients with inflammatory diseases. In inflammatory diseases, the threshold for serum ferritin is <100 µg/L or a TSAT <20%.
Source: “Anemia clarification”, 27.01.2022
Literature:
- WHO. Haemogobin concentrations for the diagnosis of anemia and assessment of severity. Vitamin and Mineral Nutrition Information System. Geneva, World Health Organization, 2011. Available at: www.who.int/vmnis/indicators/haemoglobin.pdf (last accessed on 30.03.2022)
- Zakai NA, McClure LA, Prineas R, et al: Correlates of Anemia in American Blacks and Whites: The REGARDS Renal Ancillary Study. Am J Epidemiol 2009; 169(3): 355-364.
- Beutler E, West C: Hematologic differences between African-Americans and whites: the roles of iron deficiency and α-thalassemia on hemoglobin levels and mean corpuscular volume. Blood 2005; 106(2): 740-745.
- Kassebaum NJ, Jasrasaria R, Naghavi M, et al: A systematic analysis of global anemia burden from 1990 to 2010. Blood 2014; 123(5): 615-624.
- Koury MJ, Rhodes M: How to approach chronic anemia. Hematology 2012; 1: 183-190.
- Williams TN, Weatherall DJ: World distribution, population genetics, and health burden of the hemoglobinopathies. Cold Spring Harb Perspect Med 2012; 2(9): a011692.
InFo ONCOLOGY & HEMATOLOGY 2022; 10(2): 18-19.