The use of the VEGF inhibitor bevacizumab improves the outcome of patients with metastatic colorectal cancer regardless of the chemotherapy chosen. Recent data suggest that the duration of VEGF inhibition plays an important role in treatment outcome.
The possibility of downsizing primary irresectable metastases and maximizing survival, are among the therapeutic goals of metastatic colorectal cancer (mCRC). In studies of first-line therapy for mCRC, the use of the vascular endothelial growth factor (VEGF) inhibitor bevacizumab to oxaliplatin- or irinotecan-based combination chemotherapy consistently demonstrated progression-free survival (PFS) of 10-12 months and overall survival (OS) extension of 22-28 months (Fig. 1).
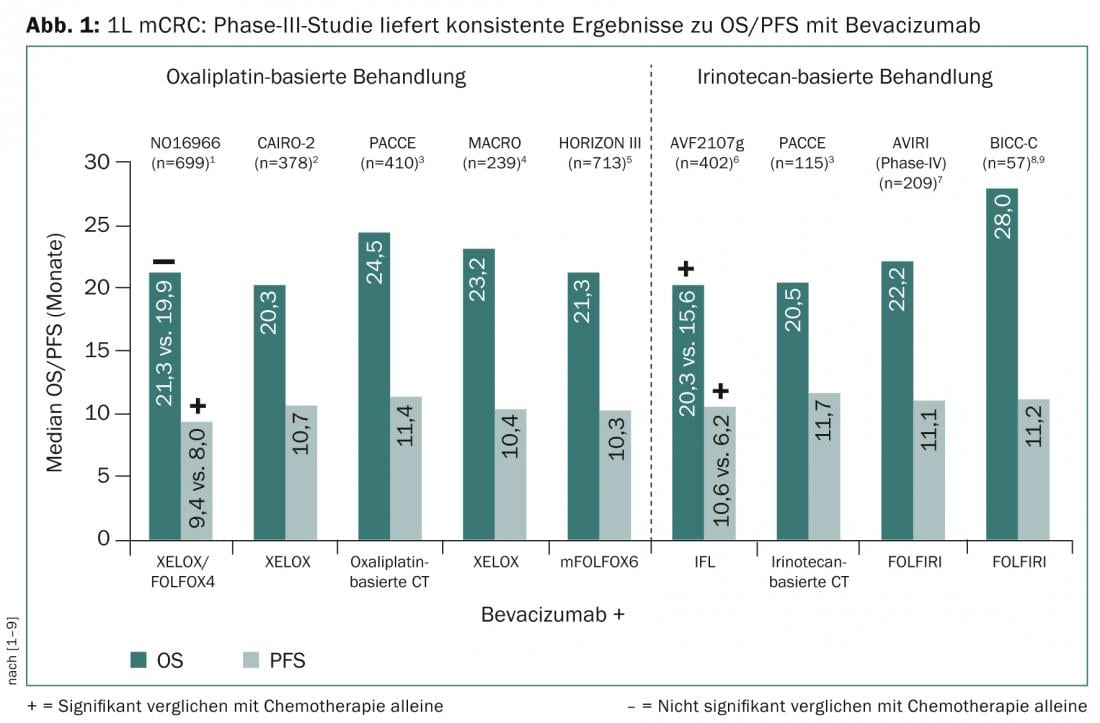
Good reasons for the use of VEGF inhibitor to monotherapy in therapy-naive mCRC were provided by the AVEX trial [10]. This showed that bevacizumab plus capecitabine resulted in a significant increase in PFS compared with capecitabine alone (9.1 months vs. 5.1; HR: 0.53; p<0.0001). “This is a big step forward and a good reason to combine less intensive therapies in particular with a VEGF inhibitor,” said Prof. Dirk Arnold, MD, Director of the Tumor Biology Clinic in Freiburg i. Br. at the 2nd EORTC St. Gallen Gastrointestinal Cancer Conference. The expert answered the question about the use of VEGF inhibitors to a triple combination based on the TRIBE results, a phase III study that compared bevacizumab in combination with FOL-FOXIRI (5-FU, folinic acid, oxaliplatin, irinotecan) or with FOLFIRI (5-FU, folinic acid, irinotecan) in first-line therapy of irresectable mCRC [11]. This showed a significant improvement in PFS (12.2 vs. 9.7 mo.; 0.73; p=0.0012) and response rate (65 vs. 53%) with the triple combination plus bevacizumab. “The TRIBE trial shows surprisingly good tolerability and the best efficacy results to date of any Phase III trial in mCRC,” Prof. Arnold said. The triple therapy strategy is supported by the data from the OLIVIA trial, which showed, among other things, twice the R0 resection rate (secondary endpoint; 48.8 vs. 23.1) with FOLFOXIRI plus bevacizumab compared with a modified FOLFOX6 regimen [12].
Data on the benefit of VEGF inhibitors in second-line therapy come from the E3200 trial of oxaliplatin-based chemotherapy (FOLFOX) plus bevacizumab, and from the VELOUR trial of aflibercept as an adjunct to FOLFIRI [13,14]. Both strategies showed a significant improvement in overall survival.
Evidence that VEGF inhibitors should be used continuously across first- and second-line therapy comes from the TML trial. This had shown that continuous administration of bevacizumab beyond progression of the first-line chemotherapy combination in combination with second-line therapy improved overall patient survival [15]. Such an approach is supported by the results of the BEBYP study and a subanalysis of VELOUR – in this case with aflibercept [16,17]. However, an indirect comparison of data from the TML and VELOUR trials showed that the adjunctive use of aflibercept resulted in a more significant increase in chemotherapy-related adverse events, whereas these were not increased with the continuation of bevacizumab [17]. Since last year, it has also been discussed whether treatment with VEGF inhibitors should be continued or interrupted during the progression-free period as “maintenance therapy”. Phase III results from the CAIRO-3 trial presented at ASCO GI in January support end-to-end treatment [18]. More information will be provided by the results of AIO 0207, the results of which will be presented at ASCO 2014.
Source: “Biologicals in colorectal cancer – state of the art”, Roche Satellite Symposium at the2nd St. Gallen EORTC (European Organisation for Research and Treatment of Cancer) Gastrointestinal Cancer Conference (GICC) 2014, March 7, 2014, St. Gallen.
Literature:
- Saltz, et al: JCO 2008; 26(12): 2013-2019.
- Tol, et al: NEJM 2009; 360(6): 563-572.
- Hecht, et al: JCO 2009; 27(5): 672-680.
- Díaz-Rubio, et al: Oncologist 2012; 17(1):15-25.
- Schmoll, et al: JCO 2012; 30(29): 3588-3595.
- Hurwitz, et al: NEJM 2004; 350(23): 2335-2342.
- Sobrero, et al: Oncology 2009; 77(2): 113-119.
- Fuchs, et al: JCO 2008; 26(4): 689-690.
- Fuchs, et al: JCO 2007; 25(30): 4779-4786.
- Cunningham D, et al: Lancet Oncol 2013; 14(11): 1077-1085.
- Falcone A, et al: JCO 2013 (Suppl. abstr 3505).
- Bridgewater J, et al: European Cancer Congress 2013. abstract 2159.
- Giantonio BJ, et al: JCO 2007; 25(12): 1539-1544.
- Van Cutsem E, et al: JCO 2012; 30(28): 3499-3506.
- Bennouna J, et al: Lancet Oncol 2013; 14(1): 29-37.
- Masi G, et al: Ann Oncol 2012; 23 (Suppl. 9): abstract LBA17.
- Carmen JA, et al: JCO 2012; 31 (Suppl. abstr 3505).
- Koopman M, et al: JCO 2013; 31 (Suppl. abstr 3502).
InFo ONCOLOGY & HEMATOLOGY 2014; 2(5): 30-31.