Schizophrenia is characterized, among other things, by dopaminergic dysregulation. All antipsychotics approved for their treatment are antagonists of dopamine receptors, particularly D2 and D3 receptors. The three partial D2 dopamine agonists aripiprazole, brexpiprazole, and cariprazine have significantly better efficacy as antipsychotics than placebo in the drug treatment of schizophrenic disorders.
The mechanism of action of currently introduced antipsychotics supports the neurobiological hypothesis that schizophrenia is characterized by, among other things, dopaminergic dysregulation [1]. All antipsychotics approved for their treatment are antagonists of dopamine receptors, particularly D2 and D3 receptors. Over the past 20 years, three new antipsychotics for the treatment of schizophrenia have been introduced in Switzerland; they belong to the group of partial D2 dopamine receptor agonists: aripiprazole, brexpiprazole, and cariprazine. Their pharmacological and clinical properties were described in detail by Eich and Gertsch in this journal in 2019 [2]. The present paper aims to update the literature published since then, but also to present some topics in more depth, with emphasis on brexpiprazole. However, recent treatment recommendations and reviews [3–5] also show that the data situation for the new representatives of this drug group, brexpiprazole and cariprazine, is still sparse compared to that of aripiprazole, which has been available for almost 20 years. It is particularly striking that direct comparisons between these three antipsychotics are extremely rare, both in terms of their clinical efficacy but also their side effect profile (tolerance, safety) [6].
Neurobiological hypotheses of schizophrenia: pharmacological aspects.
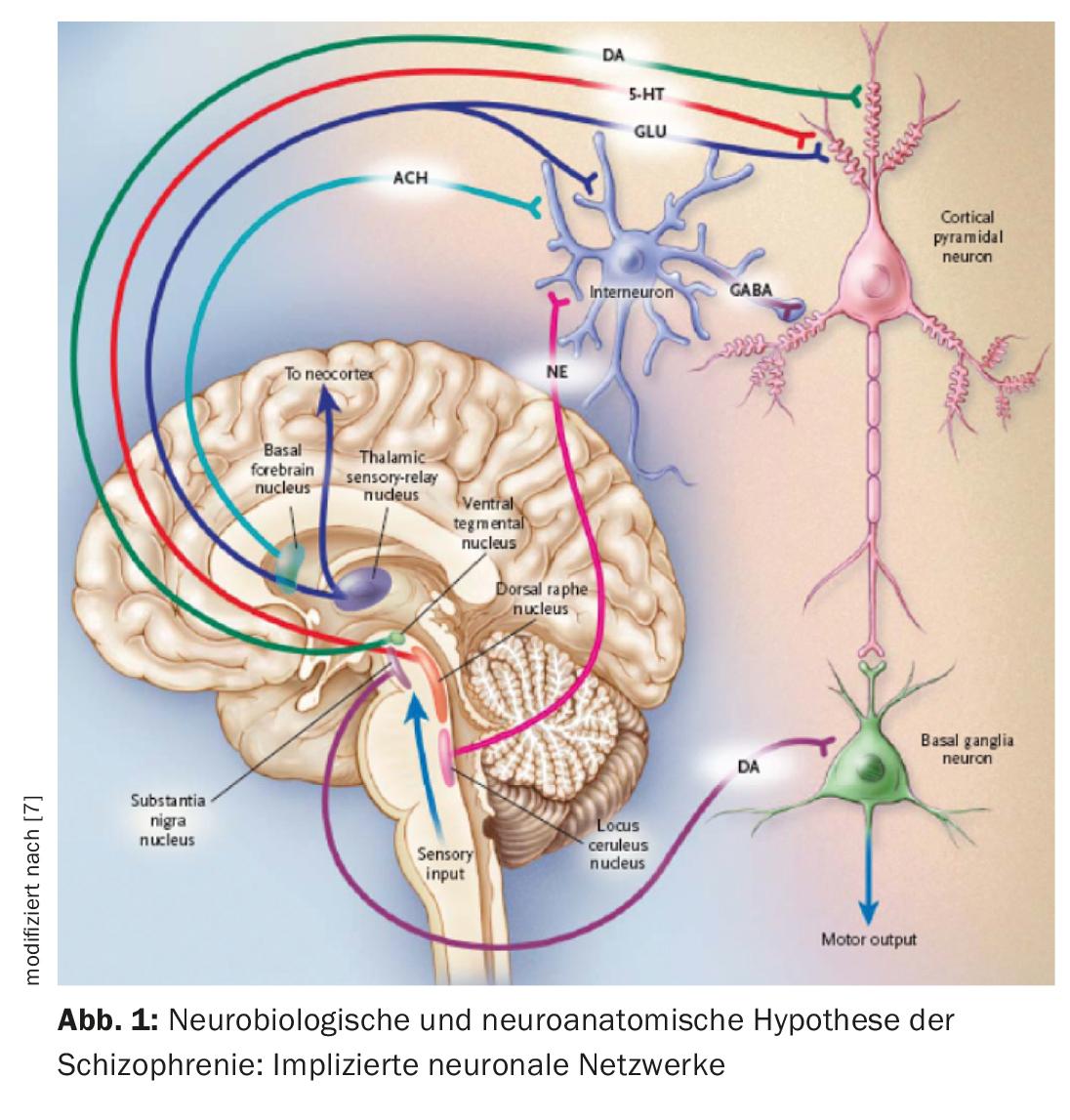
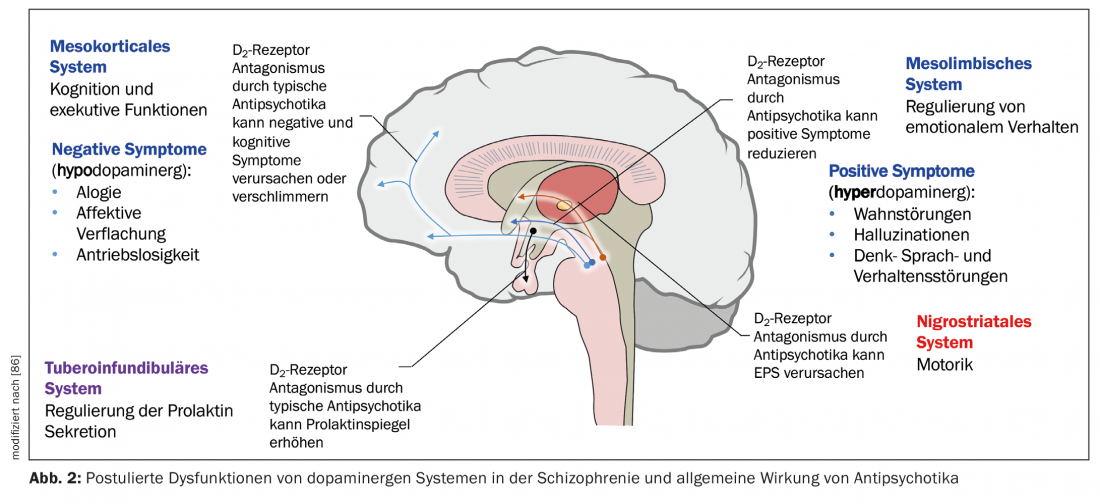
A neurobiological hypothesis of schizophrenia is based on the assumption that the thalamus no longer fulfills its role as a filtering organ for external stimuli (Fig. 1) [7]. Sensory information is conducted from thalamic nuclei to pyramidal neurons in the limbic cortex and neocortex via glutamatergic stimulatory afferents. An excessive response of pyramidal neurons could be the responsible mechanism for psychosis, consistent with hyperstimulation observed in schizophrenic patients. Different subcortical nuclei facilitate neuronal response. Dysfunction of various neurotransmitters such as glutamate, dopamine (DA), GABA, serotonin (5-HT), and norepinephrine has been postulated in relation to the ethiopathogenesis of schizophrenia [7,8]. It is possible that the glutamatergic system is mainly affected, but drugs that selectively inhibit glutamatergic neurotransmission and have been shown to be useful in the treatment of psychosis are only in the developmental stage [9]. Now, all currently available antipsychotics exhibit a dopaminergic but not all a serotonergic mechanism. DA of the ventral tegmental area activates D1 and D2 receptors, which increase the neuronal response to glutamate. Dorsal raphe nucleus serotonin activates 5-HT2A receptors, which facilitates the release of glutamate from nerve terminals. Thus, indirectly, glutamatergic activity can be lowered via an effect on 5-HT and DA. Antipsychotics generally inhibit the stimulatory effect of DA and 5-HT, including that of DA of the substantia nigra in the basal ganglia, and they thus cause motor dysfunction (Fig. 2). To specify the role of each anantomic region, there is now evidence for increased dopaminergic activity in the mesolimbic area. It would be responsible for positive or psychotic symptomatology such as hallucinations and delusions, whereas reduced activity in the mesocortical system with projections to the frontal cortex would be consistent with hypodopaminergic neurotransmission. It would account for negative symptoms such as apathy, anhedonia, social withdrawal, and poverty of thought (Fig. 2) [10].
This brief review neglects to discuss the role of D1, D3, 5-HT1a, and 5-HT2 receptors and their pharmacology [11]. It is now striking that cariprazine has a higher affinity for D3 receptors compared to aripiprazole and brexpiprazole [12,13]. Cariprazine has 10 times higher affinity for D3 than for D2 receptors. There is now a hypothesis, based on animal models, that D3 receptor antagonists favorably affect cognitive impairment by increasing dopaminergic transmission in the prefrontal cortex. In terms of mechanism, cariprazine, for example, annuls impairment triggered by the glutamate NMDA receptor antagonist phenycyclidine (PCP). However, D3 antagonism alone is not sufficient for an antipsychotic effect. On the other hand, it is recalled that certain antipsychotics such as lurasidone, amisulpride, brexpiprazole, and to a somewhat lesser extent aripiprazole are potent antagonists of 5-HT7 receptors compared to other antipsychotics including cariprazine, also a mechanism thought to explain beneficial effects on cognitive impairment [5,14,15].
Pharmacology of partial D2 dopamine receptor agonists.
Now, while most antipsychotics are pure dopamine antagonists, the partial D2 agonists have, to some extent, the property of acting as agonists when dopamine activity is low, as it is thought to be in the mesocortical system in schizophrenia, and as antagonists when dopamine activity is elevated, as it is in the mesolimbic system [10]. There are therefore authors who, perhaps in the spirit of a marketing strategy, refer to the effect of these drugs as “dopamine system stabilizers” (DSS) [16].
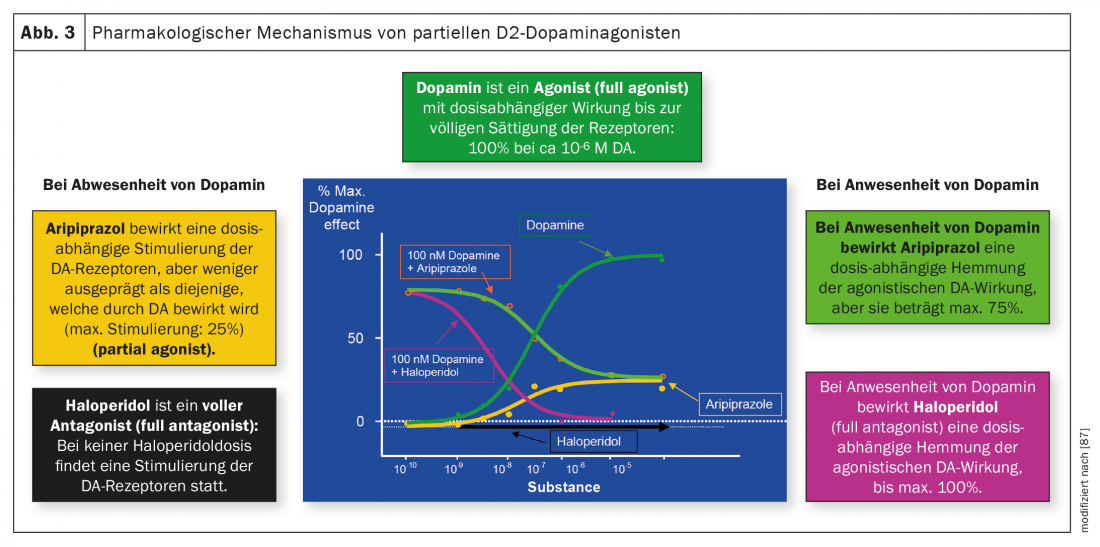
Figure 3 illustrates the differences between antagonists and partial agonists. Dopamine itself is an agonist, the classic antipsychotic haloperidol is an antagonist without any agonistic effect on dopamine receptors, while aripiprazole is agonistic on these receptors in the absence or low concentration of dopamine up to a maximum of 25%. In the presence of dopamine, haloperidol also has a purely antagonistic effect thanks to its stronger affinity for these receptors, whereas the activity of aripiprazole is antagonistic to a maximum inhibition of 75%. Brexpiprazole and cariprazine act similarly to aripiprazole. As mentioned earlier, cariprazine differs from its two other related antipsychotics in its particularly pronounced D3 receptor affinity and its partial agonist activity at this receptor, which could also explain an effect on negative symptoms and cognitive impairment.
The norepinephrine hypothesis of schizophrenia: pharmacological aspects.
Although aripiprazole, brexpiprazole, and cariprazine share the property of being partial D2 agonists, their effects on the noradrenergic system are different. The first question is whether there is evidence for its dysfunction in schizophrenic disorders and whether the therapeutic effect of antipsychotics can be explained via this neurotransmitter system. Thus, a majority of hyperactivity of the noradrenergic rather than the dopaminergic system is thought to play a role in the development of positive symptoms, whereas hypoactivity of the noradrenergic system is crucial in negative symptoms [17]. Recently, a group of authors have extensively formulated the hypothesis that the phenomenology of schizophrenia, in particular the cognitive symptoms, is explained by an abnormal interaction between genetic susceptibility and stress-induced noradrenergic dysfunction of the locus coeruleus (a core work of noradrenergic neurons) [18]. Current schizophrenia research is indeed focused on the aspects of genetics – development (prenatal and postnatal life phase, childhood, adolescence, adulthood) – stress, but it is also true that the noradrenergic hypothesis is not a focus at the moment and it should by no means be considered only in isolation from other hypotheses.
Now, however, there are also interesting pharmacological findings on the noradrenergic hypothesis of schizophrenia. A meta-analysis suggests that comedication (“augmentation”) with the α2-antagonistsmirtazapine or mianserin improves the efficacy of D2 antagonists in the treatment of schizophrenia by reducing negative symptoms [19]. According to a 2020 review, virtually all antipsychotics including aripiprazole and brexpiparzole are antagonists of α1-, α2a-, and α2b-receptors, but corresponding data are lacking for cariprazine [20]. So far, these pharmacological properties have mainly been associated with side effects. Indeed, asenapine (at α2b-receptors) and brexpiprazole (at α2c-receptors) each stand out as the most potent antagonists. Animal studies allow the hypothesis that blockade of α1-receptorscontributes to the amelioration of positive symptoms and that of α2-receptorsto the amelioration of negative and cognitive symptoms. The α2cantagonism would possibly be responsible for procognitive effects and for amelioration of anxiety and depression [21]. These receptors show a different distribution in the brain: α2-receptorsare widely distributed and more abundant (90%) than α2c-receptors(10%), which are mainly localized in the striatum, hippocampus and cortex [22].
Metabolism and pharmacokinetics of aripiprazole, brexpiprazole, and cariprazine.
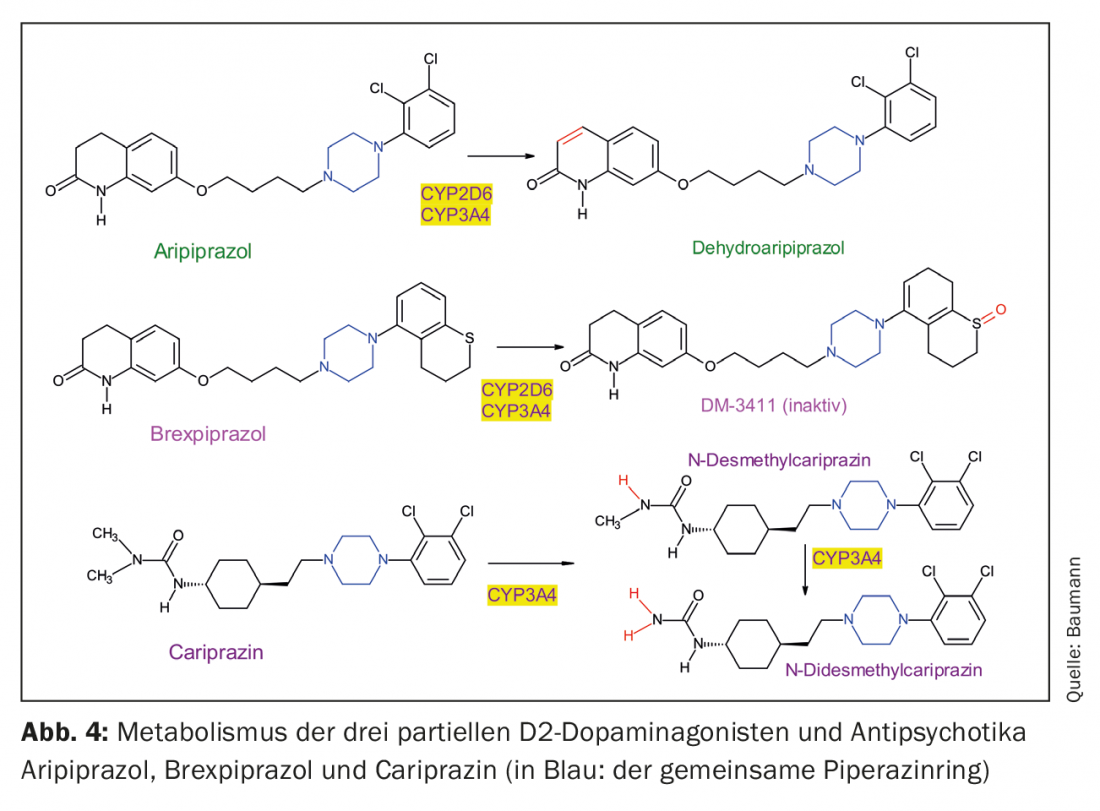
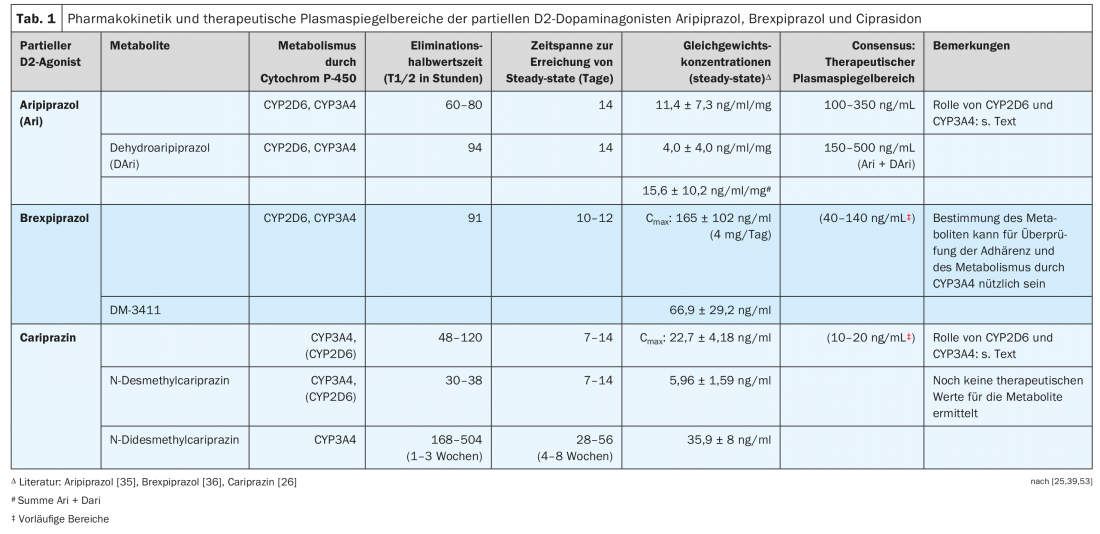
The three partial D2 agonists are structurally similar piperazine derivatives, but they differ in their metabolism (Fig. 4, Table 1). It is noticeable that cariprazine is the trans isomer of a chemical compound, with the cis isomer being less pharmacologically active [23]. Both aripiprazole and brexpiprazole do not have a structure that results in such cis-trans isomerism.
Aripiprazole is dehydrated to dehydroaripiprazole, which exerts similar pharmacological activity to its parent compound. Brexpiprazole is sulfoxidized by CYP2D6 to the metabolite (DM-3411) [24], but this is pharmacologically significantly less active than the parent compound and also does not enter the brain [24]. This was not necessarily expected since, for example, the sulfoxide metabolite of thioridazine, mesoridazine, was also marketed as an antipsychotic. The biotransformation of cariprazine is more complicated, because although the pharmacologically active desmethylcariprazine is formed via an initial N-demethylation, its equilibrium concentrations in the blood of patients treated with cariprazine are significantly lower than those of the parent substance and the next metabolite, also the product of an N-demethylation: this is the pharmacologically active N-didesmethylcariprazine, whose steady-state concentrations exceed those of cariprazine because of its particularly long elimination half-life. (Table 1) [25, 26].
Since there have been few publications on the clinical significance of the genetic polymorphism of CYP2D6 in patients treated with brexpiprazole (or cariprazine), the topic will only be briefly touched upon here. Not only aripiprazole but also its active metabolite are substrates of CYP2D6; their plasma levels strongly depend on the CYP2D6 genotype. This explains the recommendation in recent review articles and meta-analyses to consider CYP2D6 genotyping in patients treated with aripiprazole under certain conditions. This is also true for brexpiprazole, also a CYP2D6 substrate, but data are limited [27–30]. Thus, except for a Japanese one, there are no published pharmacogenetic studies on brexpiprazole: it examined only normal (extensive (EM) and intermediate (IM) metabolizers of CYP2D6 substrates, since there are hardly any pa-tients with a genetic deficiency of CYP2D6 (poor metabolizers (PM) in this group of peoples. It is also worth mentioning the one study based on a pharmacokinetic model. A simulation is used to compare the pharmacokinetics of brexpiprazole in so-called “extensive” (normal) metabolizers with the kinetics in PM as calculated by the theoretical model [31]. These studies confirm the recommendation to adjust the dose of brexpiprazole to the patient’s CYP2D6 genotype. This therefore means a prescription of only about half the usual dose in patients with a genetic deficiency or a higher dose in ultra-rapid metabolizers. The dose of either antipsychotic should also be lowered in the presence of comedications with potent CYP2D6 inhibitors such as fluoxetine or paroxetine. For all three partial D2 agonists, when comedicated with CYP3A4 inhibitors such as ketoconazole, adjust the dose downward to increase it, on the contrary, when CYP3A4-inducing drugs such as carbamazepine or certain, namely hyperforin-containing, St. John’s wort extracts are intended as comedication. Most data have been collected for aripiprazole only [32,33]. Brexpiprazole itself inhibits CYP2B6 moderately, and CYP2C9, CYP2C19, CYP2D6, and CYP3A4 only weakly, and its inhibitory effects of transporter molecules such as P-glycoprotein (P-gP) are also of little clinical relevance according to in vitro experiments. Brexpiprazole is also not a substrate of P-gP [34].
Table 1 presents examples of equilibrium concentrations in blood measured in subjects or patients during administration of one of the three D2 partial agonists [26,35,36].
Partial D2 agonists as depot preparations and their pharmacokinetics.
Depot preparations of antipsychotics have existed for decades with haloparidol decanoate, for example, by esterifying existing hydroxyl groups of the drugs with long fatty acids [37–39]. They are then dissolved in oil and administered intramuscularly to the patient as LAI (long acting injectable antipsychotic drug). This technique was no longer applicable to products such as risperidone because they do not have esterifiable OH group. A new technique was used for them by injecting such antipsychotics enclosed in microspheres. Since neither aripiprazole, brexpiprazole, nor cariprazine are hydroxy compounds (Fig. 1), a similar method would have to be developed for them. To date, there is only an LAI preparation for aripiprazole on the market, but studies are underway with the goal of also offering brexpiprazole as an LAI [40]. There are even two depot forms for aripiprazole: aripiprazole monohydrate has been introduced in Switzerland, while aripiprazole lauroxil is additionally offered in the USA [41]. It is the product of a covalent bond of lauric acid (dodecanoic acid) to a nitrogen atom of aripiprazole (Fig. 4) [42]. This must be taken into account by the treating physician for patients from abroad, as the dosages differ for the two preparations. Since the atypical depot antipsychotics often have the problem of weight and BMI gain and prolactin elevation (exception: aripiprazole) [43], it would be beneficial to also have LAIs of brexipirazole and cariprazine available [44], which have a more favorable profile in this regard as shown below.
Measurement of partial D2 agonist binding in the central nervous system by positron emission tomography (PET).
An important question is the extent to which antipsychotics are brain-responsive and the extent to which they bind to therapeutically important receptors. PET studies were initially successful in demonstrating the binding of aripiprazole to D2 and D3 receptors in the human brain [45–48]. Complete saturation of central D2/D3 receptor binding is achieved at plasma concentrations as low as 100-150 ng/mL of aripiprazole in schizophrenic patients treated with this antipsychotic [47], with a mean plasma aripiprazole concentration of 228 ng/mL (s.d.: 142 ng/mL) measured in the entire patient group (n=16) in this study.
Such PET studies have also been published for cariprazine [49] and brexpiprazole [24,50,51]. Depending on the PET ligand used, no distinction can be made between binding to D2 or D3 receptors. This technique also does not directly determine the proportion of the active metabolite upon binding, so it actually provides evidence of binding of the “active moiety” (namely, aripiprazole + dehydroaripiprazole; brexpiprazole; cariprazine + N-desmethlycariprazine + N-didesmethylcariprazine). Since brexpiprazole has no active metabolites, the observed binding refers only to the parent molecule, since, moreover, the metabolite does not enter the brain [24]. Another limitation of the PET technique is that the respective proportion of antagonism/agonism cannot be elicited directly from the data collected. Nevertheless, the PET studies provide very important findings such as the observation that there is also a dose-response curve for brexpiprazole between its plasma level and its binding to D2/D3 receptors in the striatal region, as has been reported previously for aripiprazole [45]. Four hours after a single dose (5 or 6 mg) of brexpiprazole, binding reaches 77-78% in the putamen and caudate; it remains relatively stable until 23.5 h after administration [51]. It is recalled that a binding range of 60-80% applies to an antipsychotic effect, and that stronger binding increases the risk of extrapyramidal side effects [52], which may be more the case with pure D2/D3 antagonists than with partial D2 agonists such as aripiprazole [45]. At 50% of maximum binding (Omax: approximately 90-95%), the corresponding brexpiprazole plasma levels would be approximately 8 ng/mL [51]. If the data are now extrapolated to treatment with multiple doses of 2 mg/day brexpiprazole, D2/D3 receptor binding is expected to exceed 80%, which is somewhat higher than the value reported in another PET study [50] because of the choice of a different ligand.
Relation studies: plasma levels of partial D2 agonists – clinical effect.
For aripirazole in particular, there are several studies on the relationship between its plasma levels and its clinical effects in schizophrenic patients. The consensus guidelines of the AGNP-TDM group (Working Group on Neuropsychopharmacology and Pharmacopsychiatry) [53] and those published with the American Society of Clinical Psychopharmacology [54] recommend plasma level ranges, which are shown in Table 1 . The plasma level ranges given for brexpiprazole and cariprazine must be regarded as provisional due to insufficient data. The ranges given for cariprazine do not specify whether they also include the metabolites.
Recent clinical efficacy studies of aripiprazole, brexpiprazole, and cariprazine.
In the last two years, several reviews have appeared on the clinical properties of brexpiprazole [55] and cariprazine [56,57]. Because there is now a lack of direct comparative studies between the three partial D2 agonists, network studies must be used for indirect comparisons [6,58]. A meta-analysis of the clinical efficacy of 32 oral antipsychotics found that virtually all of these drugs were significantly more beneficial than placebo [58], i.e., including aripiprazole, brexpiprazole, and cariprazine. This concerned the total score as well as the positive, negative and depressive symptomatology. Nevertheless, the presentation of the data suggests that brexpiprazole differs less than aripiprazole and cariprazine from placebo. The authors justify this by observing that over the decades, the placebo effect in such comparative studies has steadily increased, and this was particularly evident with the most recent partial D2 agonist. Brexpiprazole turned out to be better than placebo in “social functioning,” in contrast to aripiprazole, which did not differ from placebo, whereas there were no data in this regard for cariprazine. When the data were compared in terms of treatment discontinuation, aripiprazole and brexpiprazole performed better than placebo, in contrast to cariprazine. For a mutual comparison of the clinical properties of these direct antipsychotics, direct comparative studies would now be necessary, but so far these exist only to a limited extent [59,60], namely only as an open, exploratory 6-week study between aripiprazole and brexpiprazole [61].
Now, however, a study of cariprazine had attracted great attention in that it performed significantly better than risperidone for the treatment of negative symptoms in schizophrenic patients with predominant negative symptomatology [62,63]. Unfortunately, this study has not yet been replicated to confirm this important finding. In an interesting meta-analysis that included other antipsychotics, it was shown that the optimal daily doses for the treatment of negative and positive symptoms differed between drugs: Aripiprazole (11.9 mg [für die negative Symptomatik], 11 mg [für die positive Symptomatik]). The corresponding doses are for brexpiprazole: (2.1 mg, respectively 4 mg); for cariprazine: (4 mg, respectively 6.51 mg): Strictly speaking, these are the ED95 (effective dose 95%) doses at which the respective symptoms are reduced to a maximum [64]. In a similar study, ED95 was also calculated, i.e., the 95% reduction in positive symptomatology measured by the PANSS or BPRS scales compared with placebo [65]: It is 11.5 mg/day (equivalent dose to 1 mg/day risperidone: 1.8 mg/day) for aripiprazole, 3.36 mg/day (0.54 mg/day) for brexpiprazole, and 7.6 mg/day for cariprazine. The dose-response curve for brexpiprazole and cariprazine shows a plateau at higher doses, and a bell-shaped curve emerges for aripiprazole, indicating that higher doses do not promise an additional effect.
Adverse drug reactions (ADRs)
With few exceptions, the antipsychotics still in use today do not differ significantly in their clinical efficacy on schizophrenic disorders [58]. The situation is different with their side effect profiles, as presented in three remarkable reviews and network meta-analyses, respectively [58,6,25] (Tab. 2), as well as in other publications [20,66,67]. Many ADRs of antipsychotics exhibit dose dependence according to the literature, as has been documented for aripiprazole but not yet for brexpiprazole and cariprazine [68].
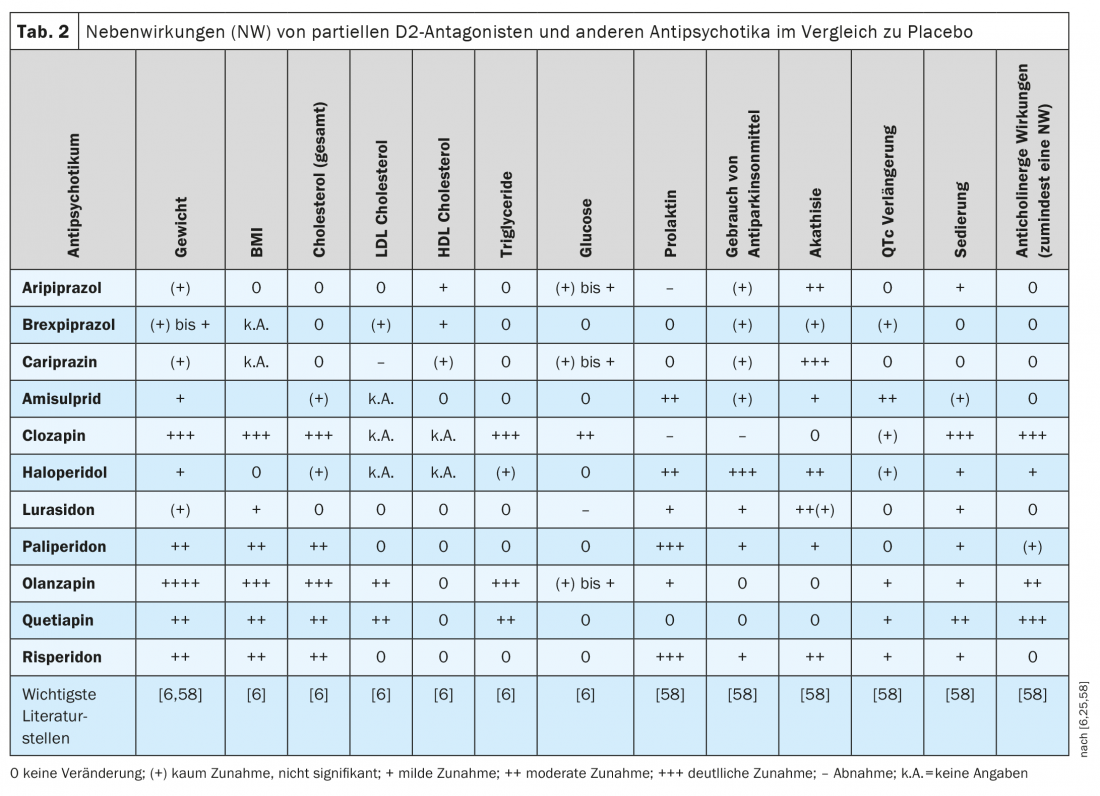
In the head-to-head comparative study between brexpiprazole and aripiprazole cited earlier, akathisia occurred less frequently with brexpiprazole (9.4%) than with aripiprazole (21.2%), but the two partial D2 inhibitors did not differ significantly in clinical effect [61], nor in measured prolactin levels. However, many patients experienced clinically significant(≥7%) weight gain after 6 weeks of pharmacotherapy in both the brexpiprazole group (35% of patients) and the aripiprazole group (19%): a finding that was confirmed in a network analysis [60] but appears rather high according to other reviews [59,69]. As shown in Table 2 based on literature, the three partial D2 agonists tend to perform well compared with many other antipsychotics in terms of weight gain or increase in BMI. There may be weight gain in some patients and weight loss in others, but this only reflects the width of the confidence interval not shown here (mostly CI: 95%), which is expressed to a greater or lesser extent in such statistical calculations from drug to drug. Aripiprazole tends to perform slightly more favorably than brexpiprazole and cariprazine [6,25,58], and in a recent retrospective study it was shown that body weight and BMI increased significantly in patients treated with brexpiprazole but not cariprazine [70]. Compared with several other 2nd generation antipsychotics, dyslipidemia occurs less frequently with the three partial D2 agonists (Table 2).
Referring again to the comparative study [61], the mean ECG showed hardly any and if so, clinically irrelevant changes under aripiprazole and brexpiprazole. Such measurements are nevertheless appropriate because QTcF increased in one patient on brexpiprazole (399 ms before treatment; 442 ms when it was discontinued). Treatment also had to be stopped in one patient on aripiprazole because of ECG changes. Further such direct comparison studies would be needed to demonstrate clear differences between these partial D2 agonists.
Compared with other antipsychotics with strong D2 antagonism, the partial D2 antagonists do not increase prolactin in the blood (Table 2) . With aripiprazole, as with clozapine, occasionally even a decrease in prolactin levels can be observed compared to placebo. Aripiprazole is therefore considered the first option to reduce elevated prolactin plasma levels in schizophrenic patients [71]. A pharmacokinetic study showed significant negative correlations between prolactin levels and plasma concentrations of aripiprazole and the sum of aripiprazole and dehydro-aripiprazole in schizophrenic patients treated with aripiprazole. Hypoprolactinemia (<8 ng/mL) occurred in several patients, although the possible clinical consequences are not clear (erectile dysfunction, decreased milk production, anxiety symptoms) [72]. On the other hand, patients on aripiprazole or cariprazine suffer more frequently from akathisia than those on brexpiprazole (Table 2).
An “augmentation” of antidepressant therapy with max. 2 mg/day brexpiprazole in depressed patients [73] who do not respond to monotherapy with antidepressants is an interesting therapeutic option [74]. However, polymedication also carries risks, as described in a report of a patient who developed hypomania after 1 week of comedication with brexpiprazole after the depressed patient had responded inadequately to combined treatment with mirtazapine and duloxetine. Strikingly, after brexpiprazole was stopped, this side effect disappeared [75]. This observation would be more or less consistent with the results of 2 studies showing no benefit of brexpiprazole compared with placebo in a treatment of bipolar mania [76].
Special clinical questions
Tardive dyskinesias: a recent meta-analysis of studies of EPS in psychiatric patients treated with antipsychotics found a high prevalence of motor side effects: EPS: 20%; akathisia: 11%; tardive dyskinesias: 7% [77]. The same group of authors reported an annual incidence of tardive dyskinesia of 0.68-6.5%. They occurred in approximately 15-30% of patients chronically treated with antipsychotics [78]. Now, antipsychotics may be prescribed rather more frequently today, namely not only for schizophrenic but also for bipolar disorders and as adjunctive medication for affective psychosis. The question is therefore urgent to what extent modern drugs such as partial D2 agonists antipsychotics can cause tardive dyskinesia. The issue of tardive dyskinesia is also topical since deutetrabenazine and valbenazine have been introduced with the indication of tardive dyskinesia in the USA but not yet in Switzerland [79]. Aripiprazole causes less tardive dyskinesia than haloperidol according to an older study [80]. A 2018 review described the occurrence of these ADRs after treatment with aripiprazole, but possibly because of their recent introduction, no such cases have yet been reported after treatment with brexpiprazole or cariprazine cases [81].
Partial D2 agonists in psychogeriatric patients: Antipsychotics generally carry a warning for their use in psychogeriatric patients suffering from dementia-associated psychosis. The risk of mortality is increased for patients treated with atypical antipsychotics (including aripiprazole) compared with placebo, that is, as a result of cardiovascular (e.g., heart failure, sudden cardiac death), infectious (e.g., pneumonia), or cerebrovascular adverse reactions. However, there are also studies on the effect of partial D2 agonists in agitated patients diagnosed with AD. For example, two 12-week placebo-controlled trials recently demonstrated satisfactory efficacy and relatively good tolerability of 2 mg/day of brexpiprazole in agitated Alzheimer’s disease patients, where the target symptom was agitation [82]. The success of this study then motivated the manufacturers of brexpiprazole to conduct further studies. A just-published review of new pharmacotherapeutic developments in psychogeriatric patients cited this study in this area [82], but none referring to aripiprazole or cariprazine [83].
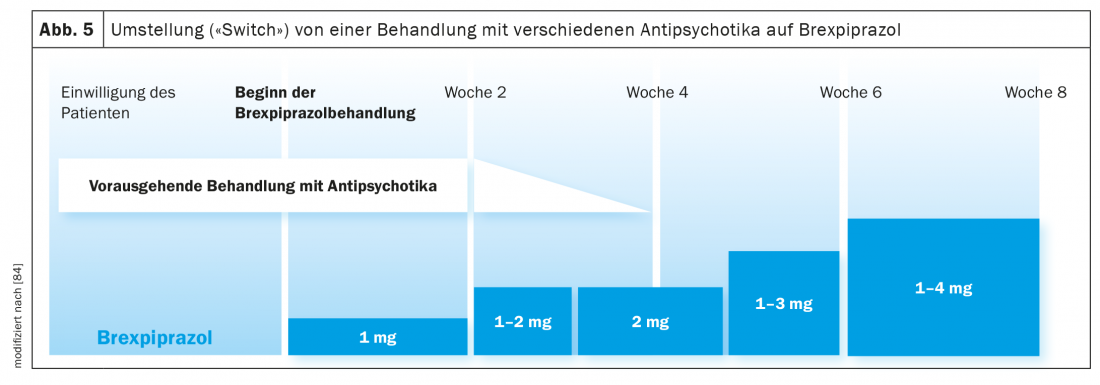
Switching of antipsychotic treatment to brexpiprazole: In a clinical study, the consequences of switching patients suffering from schizophrenia or affective psychosis from treatment with different antipsychotics to brexpiprazole were investigated (Fig. 5) [84]. The switch was realized over four weeks by maintaining the previous antipsychotic treatment for four weeks. It was slowly phased out at the beginning of week 3 to continue only brexpiprazole medication from week 4, which had been introduced at the beginning of week 1 at a low dose (1 mg/day, then 1-2 mg/day, 4 mg/day) (Fig. 5). The maximum brexpiprazole dose was 4 mg/day (in 50% of the 200 patients) but was adjusted individually according to CGI criteria. After the eighth week of study, the interruption rate reached 17%. As more or less expected, this rate was only 4.9% in patients switched from aripiprazole to brexpiprazole but 25.4% in patients treated with other antipsychotics. In summary, a changeover period of 4 weeks seems to be rather too short, especially with a premedication with e.g. olanzapine, which is characterized by anticholinergic properties. It must also be considered that the long half-lives (Table 1) of aripiprazole, brexpiprazole, and cariprazine mean that equilibrium concentrations are not reached before 10 days. In another conversion study, it was found that the introduction of brexpiprazole had a beneficial effect on preexisting EPS symptomatology. Not only was there an increase in HDL cholesterol and a decrease in body weight and BMI of the patients, but also a significant decrease in prolactin levels (the majority of patients were pretreated with risperidone or paliperidone) [85]. This beneficial effect of brexpiprazole was also seen in the patient described here (case description) after switching from risperidone to the partial D2 agonist.
Concluding remarks
Comparing the practical desired profile of antipsychotics presented by Eich and Gentsch (2019) in their review regarding their optimal safety and tolerability [2], namely: No or low EPS, no agranulocytosis, no/low weight gain, no or low sedation, no cardiotoxicity (no QTc prolongation, no hypotension), no prolactin increase/no sexual dysfunction, no/low interactions, the three partial D2 agonists aripiprazole, brexpiprazole, and cariprazine satisfactorily fulfill some of these desires. Nevertheless, after about 70 years of biologically oriented research, one would like to see a “quantum leap” in the development of efficient antipsychotic drugs. This will likely not be possible until research has given us more insight into the neurobiological causes of schizophrenic disorders, which we can then use to develop new therapeutic tools.
Take-Home Messages
- The three partial D2 dopamine agonists aripiprazole, brexpiprazole, and cariprazine have significantly better efficacy as antipsychotics than placebo in the drug treatment of schizophrenic disorders.
- In terms of safety and tolerability, they are more beneficial than many other first- and second-generation antipsychotics, particularly with regard to the use of antiparkinsonian agents, as well as increases in body weight and prolactin levels. The risk of metabolic and anticholinergic side effects is low. On the other hand, the occurrence of akathisia is particularly noteworthy during therapy with cariprazine and aripiprazole.
- Since aripiprazole and brexpiprazole are metabolized by CYP2D6, but also like cariprazine by CYP3A4, the risk of pharmacokinetic interactions (inhibition, induction) must be considered for all three antipsychotics.
- Depending on the CYP2D6 genotype of the patient, dose adjustments of aripiprazole and brexpiprazole, respectively, are recommended.
- Data are poor regarding recommendations for therapeutic monitoring (TDM) of brexpiprazole and cariprazine.
Literature:
- McCutcheon RA, Reis Marques T, Howes OD: Schizophrenia – An Overview. JAMA psychiatry 2020;77(2): 201-210.
- Eich P, Gentsch K: Similarities and differences among partial dopamine agonists. InFo NEUROLOGY & PSYCHIATRY 2019; 17(6): 16-21.
- Barnes TR, Drake R, Paton C, et al: Evidence-based guidelines for the pharmacological treatment of schizophrenia: Updated recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2020;34(1): 3-78.
- Sakurai H, Yasui-Furukori N, Suzuki T, et al: Pharmacological Treatment of Schizophrenia: Japanese Expert Consensus. Pharmacopsychiatry. 2021;54(2): 60-67.
- Frankel JS, Schwartz TL: Brexpiprazole and cariprazine: distinguishing two new atypical antipsychotics from the original dopamine stabilizer aripiprazole. Ther Adv Psychopharmacol. 2017;7(1): 29-41.
- Pillinger T, McCutcheon RA, Vano L, et al: Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta-analysis. The lancet Psychiatry. 2020;7(1): 64-77.
- Freedman R: Schizophrenia. New England Journal of Medicine. 2003; 349: 1738-1749.
- McCutcheon RA, Krystal JH, Howes OD: Dopamine and glutamate in schizophrenia: biology, symptoms and treatment. World Psychiatry. 2020; 19(1): 15-33.
- Koblan KS, Kent J, Hopkins SC, et al: A Non-D2 Receptor-Binding Drug for the Treatment of Schizophrenia. N Engl J Med. 2020;382(16): 1497-1506.
- Lieberman JA: Dopamine partial agonists: a new class of antipsychotic. CNSDrugs. 2004;18(4): 251-267.
- Kondej M, Stepnicki P, Kaczor AA: Multi-Target Approach for Drug Discovery against Schizophrenia. Int J Mol Sci. 2018;19(10).
- Stahl SM: Drugs for psychosis and mood: unique actions at D3, D2, and D1 dopamine receptor subtypes. CNS Spectr. 2017; 22(5): 375-384.
- Neill JC, Grayson B, Kiss B, et al: Effects of cariprazine, a novel antipsychotic, on cognitive deficit and negative symptoms in a rodent model of schizophrenia symptomatology. Eur Neuropsychopharmacol. 2016;26(1): 3-14.
- Risbood V, Lee JR, Roche-Desilets J, Fuller MA: Lurasidone: an atypical antipsychotic for schizophrenia. Ann Pharmacother 2012; 46(7-8): 1033-1046.
- Harvey PD, Siu CO, Hsu J, et al: Effect of lurasidone on neurocognitive performance in patients with schizophrenia: A short-term placebo- and active-controlled study followed by a 6-month double-blind extension. Eur Neuropsychopharmacol. 2013; 23(11): 1373-1382.
- Kikuchi T, Maeda K, Suzuki M, et al: Discovery research and development history of the dopamine D2 receptor partial agonists, aripiprazole and brexpiprazole. Neuropsychopharmacol Rep. 2021;41(2): 134-143.
- Yamamoto K, Shinba T, Yoshii M: Psychiatric symptoms of noradrenergic dysfunction: a pathophysiological view. Psychiatry Clin Neurosci 2014;68(1): 1-20.
- Maki-Marttunen V, Andreassen OA, Espeseth T: The role of norepinephrine in the pathophysiology of schizophrenia. Neurosci Biobehav Rev. 2020;118: 298-314.
- Hecht EM, Landy DC: Alpha-2 receptor antagonist add-on therapy in the treatment of schizophrenia; a meta-analysis. Schizophr Res. 2012; 134(2-3): 202-206.
- Siafis S, Tzachanis D, Samara M, Papazisis G: Antipsychotic Drugs: From Receptor-binding Profiles to Metabolic Side Effects. Current neuropharmacology 2018; 16(8): 1210-1223.
- Maletic V, Eramo A, Gwin K, Offord SJ, Duffy RA. The Role of Norepinephrine and Its alpha-Adrenergic Receptors in the Pathophysiology and Treatment of Major Depressive Disorder and Schizophrenia: A Systematic Review. Frontline Psychiatry. 2017(8): 42.
- Uys MM, Shahid M, Harvey BH: Therapeutic Potential of Selectively Targeting the alpha2C-Adrenoceptor in Cognition, Depression, and Schizophrenia-New Developments and Future Perspective. Front Psychiatry 2017; 8: 144.
- Yuan R-X, Xu J-W, Song L-H, et al: A convenient and practical synthesis of the cis isomer of the first orally active dopamine D3-preferring ring D3-D2 receptor partial agonist cariprazine. Synthetic Communications 2021; 51(14): 2127-2134.
- Sasabe H, Koga T, Furukawa M, et al: Pharmacokinetics and metabolism of brexpiprazole, a novel serotonin-dopamine activity modulator and its main metabolite in rat, monkey and human. Xenobiotica 2021; 51(5): 590-604.
- Keks N, Hope J, Schwartz D, et al: Comparative Tolerability of Dopamine D2/3 Receptor Partial Agonists for Schizophrenia. CNS Drugs 2020.
- Nakamura T, Kubota T, Iwakaji A, et al: Clinical pharmacology study of cariprazine (MP-214) in patients with schizophrenia (12-week treatment). Drug Des Devel Ther 2016; 10: 327-338.
- Milosavljevic F, Bukvic N, Pavlovic Z, et al: Association of CYP2C19 and CYP2D6 Poor and Intermediate Metabolizer Status With Antidepressant and Antipsychotic Exposure: A Systematic Review and Meta-analysis. JAMA psychiatry 2021; 78(3): 270-280.
- Islam F, Men X, Yoshida K, et al: Pharmacogenetics-Guided Advances in Antipsychotic Treatment. Clin Pharmacol Ther 2021; 110(3): 582-588.
- Bousman CA, Bengesser SA, Aitchison KJ, et al: Review and Consensus on Pharmacogenomic Testing in Psychiatry. Pharmacopsychiatry 2021; 54(1): 5-17.
- Carvalho Henriques B, Yang EH, et al: How Can Drug Metabolism and Transporter Genetics Inform Psychotropic Prescribing? Frontiers in genetics 2020; 11: 491895.
- Elmokadem A, Bruno CD, Housand C, Jordie EB, Chow CR, Lesko LJ, et al. Brexpiprazole Pharmacokinetics in CYP2D6 Poor Metabolizers: Using Physiologically Based Pharmacokinetic Modeling to Optimize Time to Effective Concentrations. J Clin Pharmacol. 2021.
- Waade RB, Christensen H, Rudberg I, et al: Influence of comedication on serum concentrations of aripiprazole and dehydroaripiprazole. Ther Drug Monit 2009; 31(2): 233-238.
- Castberg I, Spigset O: Effects of comedication on the serum levels of aripiprazole: evidence from a routine therapeutic drug monitoring service. Pharmacopsychiatry 2007; 40(3): 107-110.
- Sasabe H, Koga T, Furukawa M, Matsunaga M, et al: In vitro evaluations for pharmacokinetic drug-drug interactions of a novel serotonin-dopamine activity modulator, brexpiprazole. Xenobiotica 2021; 51(5): 522-535.
- Kirschbaum KM, Muller MJ, Malevani J, et al: Serum levels of aripiprazole and dehydroaripiprazole, clinical response and side effects. World J Biol Psychiatry 2008; 9(3):212-218.
- Ishigooka J, Iwashita S, Higashi K, et al. Pharmacokinetics and Safety of Brexpiprazole Following Multiple-Dose Administration to Japanese Patients With Schizophrenia. J Clin Pharmacol 2018; 58(1): 74-80.
- Correll CU, Kim E, Sliwa JK, et al: Pharmacokinetic Characteristics of Long-Acting Injectable Antipsychotics for Schizophrenia: An Overview. CNS Drugs 2021; 35(1): 39-59.
- Toja-Camba FJ, Gesto-Antelo N, Maronas O, et al: Review of Pharmacokinetics and Pharmacogenetics in Atypical Long-Acting Injectable Antipsychotics. Pharmaceutics 2021; 13(7).
- Schoretsanitis G, Baumann P, Conca A, et al: Therapeutic Drug Monitoring of Long-Acting Injectable Antipsychotic Drugs. Ther Drug Monit 2021; 43(1): 79-102.
- Wu B, Wu L, He Y, et al: Engineered PLGA microspheres for extended release of brexpiprazole: in vitro and in vivo studies. Drug development and industrial pharmacy 2021; 47(6): 1001-1010.
- Citrome L: Aripiprazole long-acting injectable formulations for schizophrenia: aripiprazole monohydrate and aripiprazole lauroxil. Expert Rev Clin Pharmacol 2016; 9(2): 169-186.
- Hard ML, Mills RJ, Sadler BM, et al: Aripiprazole lauroxil: Pharmacokinetic Profile of This Long-Acting Injectable Antipsychotic in Persons With Schizophrenia. J Clin Psychopharmacol 2017; 37(3): 289-295.
- Saucedo Uribe E, Carranza Navarro F, Guerrero Medrano AF, et al: Preliminary efficacy and tolerability profiles of first versus second-generation long-acting injectable antipsychotics in schizophrenia: A systematic review and meta-analysis. J Psychiatr Res 2020; 129: 222-233.
- de Filippis R, De Fazio P, Gaetano R, et al: Current and emerging long-acting antipsychotics for the treatment of schizophrenia. Expert Opin Drug Saf 2021; 20(7): 771-790.
- Yokoi F, Grunder G, Biziere K, et al: Dopamine D2 and D3 receptor occupancy in normal humans treated with the antipsychotic drug aripiprazole (OPC 14597): a study using positron emission tomography and [11C]raclopride. Neuropsychopharmacology 2002; 27(2): 248-259.
- Mamo D, Graff A, Mizrahi R, et al: Differential effects of aripiprazole on D(2), 5-HT(2), and 5-HT(1A) receptor occupancy in patients with schizophrenia: a triple tracer PET study.[see comment]. American Journal of Psychiatry 2007; 164(9): 1411-1417.
- Grunder G, Fellows C, Janouschek H, et al: Brain and plasma pharmacokinetics of aripiprazole in patients with schizophrenia: an [18F]fallypride PET study. Am J Psychiatry 2008; 165(8): 988-995.
- Sparshatt A, Taylor D, Patel MX, Kapur S: A systematic review of aripiprazole–dose, plasma concentration, receptor occupancy, and response: implications for therapeutic drug monitoring. J Clin Psychiatry 2010; 71(11): 1447-1456.
- Girgis RR, Slifstein M, D’Souza D, et al: Preferential binding to dopamine D3 over D2 receptors by cariprazine in patients with schizophrenia using PET with the D3/D2 receptor ligand [(11)C]-(+)-PHNO. Psychopharmacology (Berl) 2016; 233 (19-20): 3503-3512.
- Girgis RR, Forbes A, Abi-Dargham A, Slifstein M: A positron emission tomography occupancy study of brexpiprazole at dopamine D2 and D3 and serotonin 5-HT1A and 5-HT2A receptors, and serotonin reuptake transporters in subjects with schizophrenia. Neuropsychopharmacology 2020; 45(5): 786-792.
- Wong DF, Raoufinia A, Bricmont P, et al: An open-label, positron emission tomography study of the striatal D2/D3 receptor occupancy and pharmacokinetics of single-dose oral brexpiprazole in healthy participants. Eur J Clin Pharmacol 2021;77(5): 717-725.
- Founder G, Carlsson A, Wong DF: Mechanism of new antipsychotic medications – Occupancy is not just antagonism. Archives of General Psychiatry 2003; 60: 974-977.
- Hiemke C, Bergemann N, Clement HW, et al: Consensus Guidelines for Therapeutic Drug Monitoring in Neuropsychopharmacology: update 2017. Pharmacopsychiatry 2018; 51(1/2): 9-62.
- Schoretsanitis G, Kane JM, Correll CU, et al: Blood Levels to Optimize Antipsychotic Treatment in Clinical Practice: A Joint Consensus Statement of the American Society of Clinical Psychopharmacology and the Therapeutic Drug Monitoring Task Force of the Arbeitsgemeinschaft fur Neuropsychopharmakologie und Pharmakopsychiatrie. J Clin Psychiatry 2020; 81(3).
- Frampton JE: Brexpiprazole: A Review in Schizophrenia. Drugs 2019; 79(2): 189-200.
- Mucci F, Della Vecchia A, Baroni S, Marazziti D: Cariprazine as a therapeutic option for schizophrenia: a drug evaluation. Expert Opin Pharmacother 2021; 22(4): 415-426.
- Laszlovszky I, Barabassy A, Nemeth G: Cariprazine, A Broad-Spectrum Antipsychotic for the Treatment of Schizophrenia: Pharmacology, Efficacy, and Safety. Adv Ther 2021; 38(7): 3652-3673.
- Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al: Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet 2019; 394(10202): 939-951.
- Watanabe Y, Yamada S, Otsubo T, Kikuchi T: Brexpiprazole for the Treatment of Schizophrenia in Adults: An Overview of Its Clinical Efficacy and Safety and a Psychiatrist’s Perspective. Drug Des Devel Ther 2020; 14: 5559-5574.
- Kishi T, Ikuta T, Matsuda Y, et al: Aripiprazole vs. brexpiprazole for acute schizophrenia: a systematic review and network meta-analysis. Psychopharmacology (Berl) 2020; 237(5): 1459-1470.
- Citrome L, Ota A, Nagamizu K, et al: The effect of brexpiprazole (OPC-34712) and aripiprazole in adult patients with acute schizophrenia: results from a randomized, exploratory study. Int Clin Psychopharmacol 2016; 31(4): 192-201.
- Nemeth G, Laszlovszky I, Czobor P, et al: Cariprazine versus risperidone monotherapy for treatment of predominant negative symptoms in patients with schizophrenia: a randomised, double-blind, controlled trial. Lancet 2017; 389(10074): 1103-1113.
- Fleischhacker W, Galderisi S, Laszlovszky I, et al: The efficacy of cariprazine in negative symptoms of schizophrenia: post hoc analyses of PANSS individual items and PANSS-derived factors. Eur Psychiatry 2019; 58: 1-9.
- Sabe M, Zhao N, Crippa A, Kaiser S: Antipsychotics for negative and positive symptoms of schizophrenia: dose-response meta-analysis of randomized controlled acute phase trials. NPJ Schizophr 2021; 7(1): 43.
- Leucht S, Crippa A, Siafis S, et al: Dose-response meta-analysis of antipsychotic drugs for acute schizophrenia. Am J Psychiatry 2020;177(4): 342-353.
- Citrome L: The ABC’s of dopamine receptor partial agonists – aripiprazole, brexpiprazole and cariprazine: the 15-min challenge to sort these agents out. Int J Clin Pract 2015; 69(11): 1211-1220.
- Citrome L: Activating and Sedating Adverse Effects of Second-Generation Antipsychotics in the Treatment of Schizophrenia and Major Depressive Disorder: Absolute Risk Increase and Number Needed to Harm. J Clin Psychopharmacol. 2017; 37(2): 138-147.
- Yoshida K, Takeuchi H: Dose-dependent effects of antipsychotics on efficacy and adverse effects in schizophrenia. Behav Brain Res 2021; 402: 113098.
- Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al: Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet 2019.
- Greger J, Aladeen T, Lewandowski E, et al: Comparison of the Metabolic Characteristics of Newer Second Generation Antipsychotics: Brexpiprazole, Lurasidone, Asenapine, Cariprazine, and Iloperidone With Olanzapine as a Comparator. J Clin Psychopharmacol 2021; 41(1): 5-12.
- Labad J, Montalvo I, Gonzalez-Rodriguez A, et al: Pharmacological treatment strategies for lowering prolactin in people with a psychotic disorder and hyperprolactinaemia: A systematic review and meta-analysis. Schizophr Res 2020; 222: 88-96.
- Tasaki M, Yasui-Furukori N, Kubo K, et al: Relationship of Prolactin Concentrations to Steady-State Plasma Concentrations of Aripiprazole in Patients With Schizophrenia. Ther Drug Monit 2021; 43(4): 589-592.
- Thase ME, Zhang P, Weiss C, et al: Efficacy and safety of brexpiprazole as adjunctive treatment in major depressive disorder: overview of four short-term studies. Expert Opin Pharmacother 2019; 20(15): 1907-1916.
- Kishi T, Sakuma K, Nomura I, et al: Brexpiprazole as Adjunctive Treatment for Major Depressive Disorder Following Treatment Failure With at Least One Antidepressant in the Current Episode: a Systematic Review and Meta-Analysis. Int J Neuropsychopharmacol 2019; 22(11): 698-709.
- Lafreniere S, Picotte F, Legare N: Hypomania in a depressed man following the introduction of brexpiprazole: A case report. Encephale 2021;47(2): 185-186.
- Vieta E, Sachs G, Chang D, et al: Two randomized, double-blind, placebo-controlled trials and one open-label, long-term trial of brexpiprazole for the acute treatment of bipolar mania. J Psychopharmacol 2021; 35(8): 971-982.
- Ali T, Sisay M, Tariku M, et al: Antipsychotic-induced extrapyramidal side effects: A systematic review and meta-analysis of observational studies. PLoS One 2021; 16(9): e0257129.
- Ali Z, Roque A, El-Mallakh RS: A unifying theory for the pathoetiologic mechanism of tardive dyskinesia. Med Hypotheses 2020; 140: 109682.
- Bashir HH, Jankovic J: Treatment of tardive dyskinesia. Neurol Clin 2020; 38: 379-396.
- Amada N, Akazawa H, Ohgi Y, et al: Brexpiprazole has a low risk of dopamine D2 receptor sensitization and inhibits rebound phenomena related to D2 and serotonin 5-HT2A receptors in rats. Neuropsychopharmacol Rep 2019; 39(4): 279-288.
- Carbon M, Kane JM, Leucht S, Correll CU: Tardive dyskinesia risk with first- and second-generation antipsychotics in comparative randomized controlled trials: a meta-analysis. World Psychiatry 2018; 17(3): 330-340.
- Grossberg GT, Kohegyi E, Mergel V, et al: Efficacy and Safety of Brexpiprazole for the Treatment of Agitation in Alzheimer’s Dementia: Two 12-Week, Randomized, Double-Blind, Placebo-Controlled Trials. Am J Geriatr Psychiatry 2020; 28(4): 383-400.
- Aftab A, Lam JA, Liu F, et al: Recent developments in geriatric psychopharmacology. Expert Rev Clin Pharmacol 2021; 14(3): 341-355.
- Ishigooka J, Usami T, Iwashita S, et al: Post-hoc analysis investigating the safety and efficacy of brexpiprazole in Japanese patients with schizophrenia who were switched from other antipsychotics in a long-term study (Secondary Publication). Neuropsychopharmacol Rep. 2020;40(2): 122-129.
- Ichinose M, Miura I, Horikoshi S, et al: Effect of Switching to Brexpiprazole on Plasma Homovanillic Acid Levels and Antipsychotic-Related Side Effects in Patients with Schizophrenia or Schizoaffective Disorder. Neuropsychiatr Dis Treat 2021; 17: 1047-1053.
- Stahl SM: Stahl’s essential psychopharmacology: neuroscientific basis and practical application.4th ed. New York, USA: Cambridge University Press; 2013: 626.
- Bandelow B, Meier A: Aripiprazole, a “dopamine-serotonin system stabilizer” in the treatment of psychosis. German Journal of Psychiatry 2003; 6: 9-16.
InFo NEUROLOGY & PSYCHIATRY 2021; 19(6): 12-23.