Resistance to antibiotic substances is increasing worldwide. This leads to a gradual loss of efficacy of many antibiotics and makes the treatment of many infections more difficult. Resistance to β-lactam antibiotics in Gram-negative bacteria is often associated with the production of β-lactamases. Some of the efforts to overcome this common resistance mechanism have been successful. In addition to already approved substances, several active ingredients are currently being investigated in clinical trials.
The increasing antibiotic resistance of bacterial pathogens is a major problem. This affects both inpatient and outpatient care settings, especially since approximately 80% of antibiotics are prescribed in the outpatient setting [1,2]. Strategies to combat antibiotic resistance can be implemented as part of antibiotic stewardship programs or independently. The Swiss Society of Infectious Diseases publishes guidelines on various types of infections, and numerous projects have been launched on antibiotic resistance (box) [3]. “Multidrug-resistant Gram-negative bacteria remain the most difficult to treat because there are few good options for them,” explained Prof. Sarah Tschudin Sutter, MD, Clinic for Infectious Diseases & Hospital Hygiene at University Hospital Basel [4]. Carbapenem-resistant Acinetobacter baumannii, carbapenem-resistant Pseudomonas aeruginosa, and third-generation cephalosporin-resistant pathogens were ranked by WHO as “top-ranking pathogens” in the priority list for new antibiotic development.
|
The Swiss Center for Antibiotic Resistance (ANRESIS), a national surveillance system and research unit on antibiotic resistance and antibiotic use, provides, among other things, an interactive display of the latest resistance data at [15]. The Department of Home Affairs and the Department of Economic Affairs, Education and Research are responsible for implementing the national antibiotic resistance strategy (StAR) [17]. StAR pursues the goal of preserving the efficacy of antibiotics for humans and animals in the long term. Among other things, specific recommendations are also made regarding hygiene measures to improve kitchen hygiene in order to prevent the transmission of antibiotic-resistant bacteria through plant or animal foods. This is in line with the World Health Organization’s (WHO) “One Health” approach to combating antibiotic-resistant bacteria [18]. A “Surveillance ATLAS of Infectious Diseases” is offered by the European Center for Disease Control (ECDC) on its homepage. This tool makes it possible to survey antibiotic resistance in different countries for numerous important bacterial pathogens [16]. Research into resistance mechanisms and the development of new antibiotics is being carried out in numerous study projects around the world. |
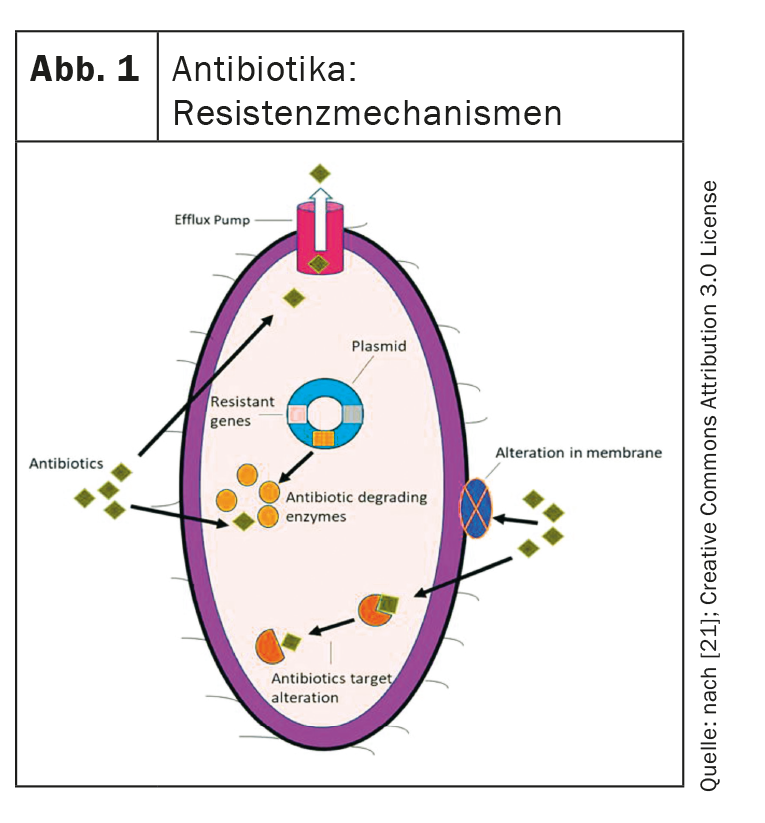
The main resistance mechanisms and “unmet needs”.
These are the four main resistance mechanisms that bacteria can express and that new antibiotics must overcome (Fig. 1) [4,21,22]:
- Porin loss: Mutations in porin genes lead to porin loss and thus to reduced permeability of the bacterial cell membrane. This hinders the absorption of the antibiotics.
- Efflux pumps: Efflux pumps are also an important factor. These transport antibiotics to extracellular and can be upregulated.
- Target modification: The binding site of the antibiotics can be modified in such a way that the antibiotics can no longer exert their effect.
- Hydrolyzing enzymes: These can enzymatically degrade the antibiotics.
The majority of currently available antibiotic substances are substances produced by fungi and bacteria themselves, with only a few synthetically produced substances. In the last 10-15 years, hardly any new antibiotics have come onto the market. In the context of evolving resistance to antibiotics, there is an urgent need for the development of new antibiotic agents [5].
Resistance to β-lactamases: a worldwide problem
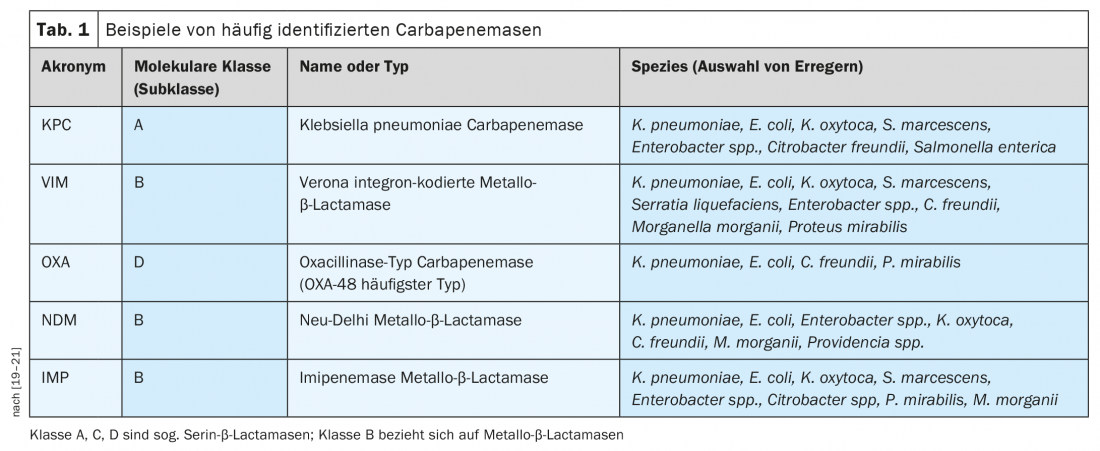
The World Health Organization (WHO) has issued a statement on prioritizing antibiotic development [6]. This indicates that there is a great need for antibiotics with efficacy in Gram-negative pathogens to combat carbapenem resistance in particular [6]. Resistance to β-lactam antibiotics in Gram-negative bacteria is often associated with the production of β-lactamases, including extended-spectrum β-lactamases ( ESBLs) and carbapenemases (Table 1), which belong to different molecular classes [7]. Carbapenemases are bacterial enzymes whose development of resistance to antibiotics poses an existing or potential threat to public health [8]. The group of ESBLs is homogeneous, meaning they have the same substrates, which is an advantage treatment-wise. In contrast, carbapenemases are a very heterogeneous group of enzymes with different chemical properties and different substrates. “Accordingly, it is hardly possible to find substances that are effective for all the different classes of carbapenemases,” she said [4]. The various carbapenemases are widespread globally, including in Europe. In particular, carbapenem-resistant Pseudomonas aeruginosa (CRPA) and carbapenem-resistant Acinetobacter baumannii (CRAB) can express different resistance mechanisms simultaneously.
What are the solution strategies?
Many of the substances that have come onto the market in recent years are modifications of already available antibiotics or combinations of β-lactams with β-lactamase inhibitors. Unfortunately, there is no new class of antibiotics, Prof. Tschudin Sutter said. In terms of β-lactams, cefiderocol – a cephalosporin with a new mechanism of action that was approved by the European Medicines Agency (EMA) in 2020 for the treatment of infections caused by aerobic Gram-negative pathogens in adults – is worth mentioning. [9]. Cefiderocol is structurally a cephalosporin to which a side chain with a catechol structure has been coupled. Side chains in the molecule increase stability against β-lactamases. The so-called siderophore cephalosporin cefiderocol is characterized by its ability to bind trivalent iron. Aerobic Gram-negative bacteria need iron and have an active transport system for iron-loaded siderophores to meet their needs. Cefiderocol uses this to pass through the outer cell membrane and then destroy the cell by disrupting cell wall synthesis [10]. Cefiderocol exhibits high hydrolytic stability to virtually all β-lactamases, including extended spectrum beta-lactamases (ESBL), AmpC enzymes, and serine and metallo-carbapenemases.
Promising results are also available for the oral antibiotic tebipenem. In a phase III study published in the New England Journal of Medicine , tebipenem (oral) was found to be non-inferior to ertapenem (i.v.) for the treatment of complicated urinary tract infections and showed a comparable side effect profile [11].
New therapeutic options that can overcome resistance are also needed against the Gram-negative pathogen Pseudomonas aeruginosa. The most common mechanisms of resistance to β-lactam antibiotics occurring in P. aeruginosa are increased expression of intrinsic β-lactamases, acquisition of new β-lactamases, increased efflux by efflux pumps, and loss or decreased expression of porins in the cell membrane, which reduces or prevents antibiotic uptake [12]. Among others, patients with cystic fibrosis require effective antibiosis against chronic infections with P. aeruginosa. Inhaled levofloxacin showed a trend toward improvement in pulmonary function and a longer time interval to exacerbation in this patient population compared with tobramycin [13].
Delafloxacin is also a quinolone and was approved in Switzerland in 2020 as a reserve antibiotic for the treatment of acute bacterial skin and skin structure infections (ABSSSI) [14] The effects are based on inhibition of bacterial topoisomerase IV and DNA gyrase. The fluoroquinolone has bactericidal properties against Gram-positive and Gram-negative bacteria and is also effective against problem germs such as Klebsiella pneumoniae, MRSA, Pseudomonas aeruginosa. Delafloxacin has anionic character at neutral pH and is mainly in uncharged form at slightly acidic pH, which is a difference from other fluoroquinolones, which are present as cations at acidic pH and mainly as zwitterions at higher values, and whose activity decreases in acidic environments [23].
Congress: medArt
Literature:
- Weber R, Chmiel C: Infectiology – Therapy Recommendations, Updated on: 12/2021, www.medix.ch/media/gl_infektiologie_therapieempfehlungen_06.2021_23.6.21_mh_1.pdf, (last accessed 08/31/2022).
- FOPH: How are antibiotics prescribed in Switzerland today? www.bag.admin.ch/bag/de/home/krankheiten/infektionskrankheiten-bekaempfen/antibiotikaresistenzen/wie-werden-heute-in-der-schweiz-antibiotika-verschrieben.html, (last accessed Aug. 31, 2022)
- Swiss Society of Infectious Diseases, www.ssi.guidelines.ch, (last accessed Aug. 31, 2022).
- “New Antibiotics”, Prof. Dr. med. Sarah Tschudin Sutter, medArt 20.-24.06.2022
- BAG: Framework conditions in the field of antibiotics, www.bag.admin.ch/bag/de/home/strategie-und-politik/nationale-gesundheitsstrategien/strategie-antibiotikaresistenzen-schweiz/rahmenbedingungen-im-bereich-der-antibiotika.html, (last accessed Aug. 31, 2022).
- Tacconelli E, et al: Discovery, research, and develop ment of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 2018; 18: 318-327.
- Bush K, Bradford PA: Interplay between β-lactamases and new β-lactamase inhibitors. Nat Rev Microbiol 2019; 17(5): 295-306.
- BAG: Carbapenemase-producing Enterobacteriaceae (CPE), www.bag.admin.ch/bag/de/home/krankheiten/krankheiten-im-ueberblick/antibiotikaresistente-bakterien.html, (last accessed Aug. 31, 2022).
- Drug Information, www.ema.europa.eu/en/documents/product-information/fetcroja-epar-product-information_de.pdf, (last accessed Aug. 31, 2022).
- Rössler A: “Cefiderocol: New antibiotic to overcome resistance”, Pharmazeutische Zeitung, 19.01.2021.
- Eckburg PB, et al: Oral tebipenem pivoxil hydrobromide in complicated urinary tract infection. N Engl J Med 2022; 386(14): 1327-1338.
- Poole K: Pseudomonas aeruginosa: Resistance to the max. Front Microbiol 2011(2): 1-13.
- Elborn JS, et al: A phase 3, open-label, randomized trial to evaluate the safety and efficacy of levofloxacin inhalation solution (APT-1026) versus tobramycin inhalation solution in stable cystic fibrosis patients. J Cyst Fibros 2015; 14(4): 507-514.
- Drug Information, www.swissmedicinfo.ch/default.aspx, (last accessed Aug. 31, 2022).
- ANRESIS, www.anresis.ch/de, (last accessed Aug. 31, 2022).
- European Centre for Disease Prevention and Control (ECDC), www.ecdc.europa.eu/en/surveillance-and-disease-data, (last accessed Aug. 31, 2022).
- Antibiotic Resistance Strategy Switzerland (StAR), https://www.blv.admin.ch/blv/de/home/das-blv/strategien/nationale-strategie-antibiotikaresistenzen.html, (last accessed Aug. 31, 2022).
- Walsh TR: A one-health approach to antimicrobial resistance. Nat Microbiol. 2018; 3: 854-855.
- Magiorakos AP, et al: Infection Prevention and Control Measures and Tools for the Prevention of Entry of Carbapenem-Resistant. Antimicrob Resist Infect Control 2017; 6: 113. https://dx.doi.org/10.1186/s13756-017-0259-z.
- Tzouvelekis LS, et al: Carbapenemases in Klebsiella Pneumoniae and Other Enterobacteriaceae: An Evolving Crisis of Global Dimensions. Clin Microbiol Rev 2012; 25(4): 682-707. https://dx.doi.org/10.1128/CMR.05035-11.
- Swissnoso, www.swissnoso.ch/fileadmin/swissnoso/Dokumente/5_Forschung_und_Entwicklung/8_Swissnoso_Publikationen/211115_StAR_Teil_II_DE_MDRO-non-outbreak_FINAL.pdf, (last accessed Aug. 31, 2022).
HAUSARZT PRAXIS 2022; 17(9): 20-21