Children and adolescents affected by psoriasis and/or psoriatic arthritis often suffer a great deal. Particularly in cases of severe symptoms, the quality of life of those affected and their families is often significantly reduced. For this patient population, effective and tolerable systemic therapies are an important component of individual disease management.
Secukinumab binds to the cytokine interleukin-17A (IL-17A) and inhibits interaction with its receptor. Thus, the active ingredient develops anti-inflammatory and immunomodulating effects. Cosentyx® (secukinumab) has been approved for adults in Switzerland since 2015 for the systemic treatment of plaque psoriasis and has now also received approval for the treatment of psoriatic arthritis. Recently, the anti-IL17A antibody has been shown to be an effective and safe treatment option also in pediatric patients with psoriasis. In this patient population, there is a need for an expansion of the treatment spectrum. “Living with psoriasis is challenging and can be very stressful for children and adolescents,” said Randy Beranek, president and CEO of the National Psoriasis Foundation [1]. “Expanding treatment options for this patient population is a step in the right direction to reduce the disease burden of plaque psoriasis” [1].
EMA and Swissmedic: approval of Cosentyx® for juvenile plaque psoriasis
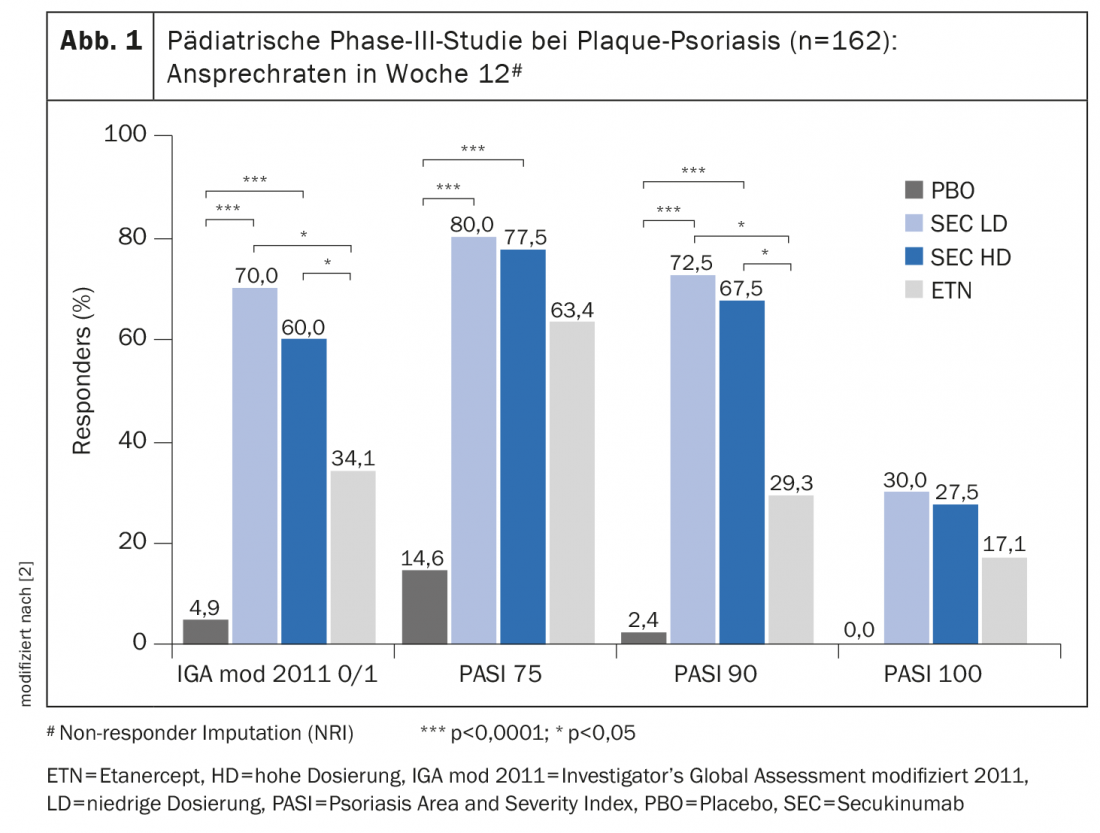
When treating children and adolescents, it is particularly important to achieve the best possible balance between the benefits and risks of system therapy. Secukinumab has been studied, among others, in a randomized double-blind clinical trial (NCT02471144) in 162 children and adolescents aged 6 to <18 years with severe plaque psoriasis [2]. Secukinumab achieved significantly better efficacy compared with placebo and etanercept (box). The safety of secukinumab was investigated in this and another phase III study. The second study was an open-label study in 84 patients (age range also 6 to <18 years) with moderate to severe plaque psoriasis [1]. The safety profile reported in these two studies in a total of 2874 patient-years was consistent with the safety signals of secukinumab reported in adult patients with plaque psoriasis [3]. “In the pivotal pediatric study, the majority of patients treated with Cosentyx® achieved lesion-free or nearly lesion-free skin with a safety profile consistent with previous clinical trials in adults. Due to the systemic nature of this disease, Cosentyx® is a welcome complementary treatment option for families facing this challenging condition,” said John Browning, MD, Assistant Professor of Pediatrics and Dermatology, University of Texas Health Science Center, San Antonio (USA) [1].

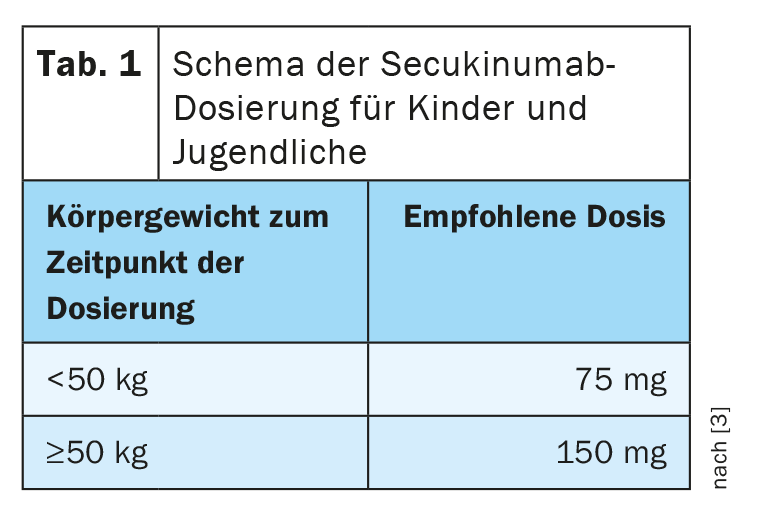
In Switzerland, the indication extension for the treatment of plaque psoriasis in children and adolescents aged 6 years and older took place in January 2021. This decision followed the European Commission’s approval of secukinumab for first-line treatment of moderate to severe plaque psoriasis in children and adolescents a few months earlier. The recommended dose depends on body weight (Table 1) and is administered as a subcutaneous injection with starting doses at weeks 0, 1, 2, 3, and 4, followed by monthly maintenance doses [3]. Each 75 mg dose is administered as a 75 mg subcutaneous injection and each 150 mg dose is administered as a 150 mg subcutaneous injection.
FDA gives green light for use in juvenile psoriatic arthritis (JPsA)
IL-17A is not only relevant for the pathogenesis of plaque psoriasis, but also plays a key pathogenetic role in psoriatic arthritis (PsA). In Switzerland, Cosentyx® (secukinumab) has so far only been approved for adults with PsA who are eligible for systemic therapy [3]. As remarkable treatment successes had also been achieved in the patient population of pediatric patients with PsA in Phase III clinical trials, a marketing authorization application for an indication extension was submitted to the European Medicines Agency (EMA) last year. In the USA, Cosentyx® has already been approved by the FDA for the treatment of children and adolescents with enthesitis-related arthritis (ERA) and psoriatic arthritis (PsA) at the end of 2021 [4]. In the pivotal clinical trial JUNIPERA, a reduction in the risk of acute relapses as well as an improvement in disease activity with respect to both ERA and PsA was demonstrated in pediatric patients over an observation period of 2 years in a placebo comparison. The safety profile was consistent with the safety signals of secukinumab reported in previous studies [4,5]. Cosentyx® has been approved by the FDA for children and adolescents with ERA and PsA, respectively, at a weight-adjusted dose of 75 mg (15-50 kg bw) or 150 mg (>50 bw), as a subcutaneous injection every 4 weeks, following an initial loading dose. “The findings from the Phase III JUNIPERA study show that pediatric patients treated with secukinumab demonstrated a significant response throughout the study period. This approval is welcome news for patients who struggle with painful symptoms such as joint inflammation and swollen fingers and toes,” said Hermine Brunner, MD, Cincinnati Children’s Hospital (USA) [4]. “Symptoms of PsA and ERA can be burdensome for children and adolescents with these chronic conditions and interfere with their daily lives,” said Tiffany Westrich-Robertson, CEO, International Foundation for Autoimmune & Autoinflammatory Arthritis (AiArthritis). “It is encouraging that there is now a complementary treatment option for this underserved patient population.”
Literature:
- “Novartis Cosentyx receives FDA approval for treatment of children and adolescents with moderate to severe plaque psoriasis,” Novartis, June 01, 2021.
- Bodemer C, et al: Secukinumab Demonstrates High Efficacy and a Favourable Safety Profile in Paediatric Patients with Severe Chronic Plaque Psoriasis: 52-week Results from a Phase 3 Double-Blind Randomized, Controlled Trial. J Eur Acad Dermatol Venereol 2021; 35: 938-947. doi: 10.1111/jdv.1700
- Swiss Drug Compendium, https://compendium.ch, (last accessed 07.04.2022).
- “Novartis Cosentyx® receives FDA approval for the treatment of children and adolescents with enthesitis-related arthritis and psoriatic arthritis,” Novartis, Dec. 23, 2021.
- Menter A, et al: Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis in pediatric patients. J Am Acad Dermatol 2020; 82(1): 161-201.
DERMATOLOGIE PRAXIS 2022; 32(2): 36-37