The prospective, multicenter observational study SURE Switzerland investigated the efficacy of once-weekly subcutaneously administered semaglutide in patients with type 2 diabetes in clinical practice. Among other things, it was shown that study participants achieved significant reductions in HbA1c levels and body weight, consistent with the pattern of results from the SUSTAIN randomized-controlled trial program.
In accordance with the American Diabetes Association (ADA) and European Association for the Study of Diabetes (EASD) guidelines, the Swiss Society of Endocrinology and Diabetology (SGED) recommends an individualized treatment approach for type 2 diabetes (T2D) [2–5]. Selection of the appropriate antidiabetic drug should include patient preferences, cardiovascular risk, influence on body weight, and side effect risks in addition to efficacy and hypoglycemia risk [2–5]. The 2020 SGED guidelines advise early use of a GLP-1 receptor agonist (GLP-1-RA) or SGLT-2 inhibitor as second-line therapy in combination with metformin to reduce the cardiovascular burden in T2D [5]. The long-acting GLP-1 receptor agonist semaglutide is available as a once-weekly injection (Ozempic®) and, more recently, as a once-daily oral formulation (Rybelsus®) [6].
SURE Switzerland: prospective study in an everyday setting.
The international SURE study program is conducted as an adjunct to the SUSTAIN randomized controlled trial program. The SURE Switzerland study is the Swiss sub-study (box) [1]. The RCTs of the SUSTAIN program, which included more than 8000 patients, also investigated the subcutaneous administration of semaglutide in T2D. In addition to a reduction in HbA1c levels, patients on semaglutide treatment achieved a reduction in body weight and those at increased cardiovascular risk were shown to have a reduction in cardiovascular events [7].

For the SURE Switzerland study, patients were recruited in 28 centers in Switzerland, and among the medical practitioners were 8 general practitioners and 24 specialists. The first visit took place in September 2018, and the last one was in December 2019. The duration of the study was 28-38 weeks. Included were ≥18-year-old type 2 diabetic patients with documented HbA1c values. A BMI of ≥28 kg/m2 is considered a prerequisite for reimbursement of semaglutide by health insurance in Switzerland [5]. At baseline, 214 patients* with type 2 diabetes were included The mean age was 60.2 years, and the mean duration of existing diabetes was 11 years. Mean HbA1c was 7.8% [62 mmol/mol], mean body weight was 99.9 kg, and mean hip circumference was 117.4 cm.
* Full Analyses Set (FAS)
The treating physician determined the initial dose of semaglutide treatment, and this also applied to dose escalation and any changes in maintenance dose. Counseling regarding dietary changes and exercise, as well as regarding other pharmacologic antihyperglycemic therapies, was also at the discretion of the treating physician**.
** The SGED 2020 guideline recommends the use of GLP-1RA in combination with metformin [5].
The primary endpoint of the study was the change in HbA1c levels from baseline to study end (weeks 28-38). Secondary endpoints included changes in body weight and hip circumference and the proportion of patients who achieved HbA1c values <8.0%, <7.5%, and <7.0% at the end of the study.
Results broadly similar to those of the SUSTAIN RCTs.
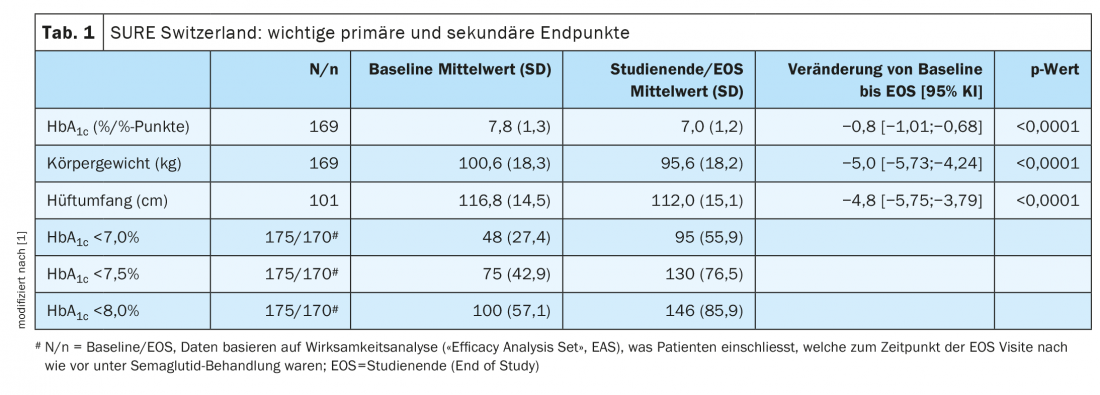
Both the primary and several secondary endpoints of the SURE Switzerland study were met. As shown in Table 1 , the following changes since baseline were found to be significant at the end of the study (weeks 28-38):
- average HbA1c reduction of 0.8% points, corresponding to a reduction of 9 mmol/mol
- Mean weight loss by 5.0 kg
- Mean reduction in hip circumference of 4.8 cm .
Furthermore, 85.9%, 76.5% resp. 55.9% of patients HbA1c <8.0%, <7.5% resp. <7.0% .
That a reduction in HbA1c levels and body weight was achieved is consistent with the results of the SUSTAIN study. Smaller deviations in the extent of the changes can be explained by differences in methodological aspects. Reductions in HbA1c levels were slightly lower in SURE Switzerland, at 0.8% points, than in the SUSTAIN RCTs, where these levels ranged from 1.1% points with 0.5 mg semaglutide in SUSTAIN 6 to 1.8% points with 1.0 mg marine aglutide in SUSTAIN 5 and 7 [8–10]. The authors attribute this, among other things, to lower mean HbA1c values at baseline. Thus, in the SURE Switzerland study, these were 7.8% while in the SUSTAIN studies they were in the range of 8.0-8.7%. In the SURE Switzerland study, there were no constraints on baseline HbA1c values, whereas in the SUSTAIN studies, HbA1c values ≥7.0 resp. 7.5% were among the inclusion criteria. The dosage of semaglutide is another influencing factor. At the last follow-up measurement point of the SURE Switzerland study, a dose <1mg was documented in approximately 40% of patients, whereas in the SUSTAIN studies the initial and maintenance doses of semaglutide had been established a priori with dose escalation steps from 0.25 mg to 0.5 mg and to 1.0 mg. (The officially recommended initial dose of semaglutide is 0.25 mg, with maintenance doses of 0.5 mg after 4 weeks and 1.0 mg after an additional 4 weeks as needed) [11]. Another difference with the SUSTAIN program was that in the SURE Switzerland study, 22.4% of patients had had previous treatment with a GLP-1 RA at baseline and were subsequently switched to semaglutide, whereas in SUSTAIN all patients were GLP-1 RA-naive before initiation of semaglutide treatment.
The average weight reduction in the SURE Switzerland trial proved to be similar to that in the SUSTAIN trials. In SURE Switzerland, a mean weight loss of 5.0 kg (-5.0%) was achieved, compared with 3.5 kg (-3.8%) in SUSTAIN 4 with 0.5 mg semaglutide and 6.5 kg (-6.9%) in SUSTAIN 7 with 1.0 mg semaglutide [9,12].
Overall, the improvements in glycemic control and weight loss demonstrated in the randomized-controlled SUSTAIN trial program were replicated in the SURE Switzerland study in the clinical setting and in the “real world” setting, respectively. The differences in the magnitude of change can be explained by implications of the different study designs. No new safety signals appeared in the SURE Switzerland study.
Satisfaction with therapy was assessed in the SURE Switzerland study using the Diabetes Treatment Satisfaction Questionnaire (DTSQ), again showing a significant improvement in scores since baseline at the end of the study (p<0.0001). The DTSQ is a validated self-report tool that measures the three dimensions of treatment satisfaction, hyper- and hypoglycemia perception using 8 items [13]. The application time is about 5-10 minutes, the evaluation about 5 minutes.
Literature:
- Rudofsky G, et al: Real-world use of once-weekly semaglutide in patients with type 2 diabetes: results from the SURE Switzerland multicentre, prospective, observational study. Diabetes Res Clin Pract. 2021;178: 108931. doi: 10.1016/j.diabres.2021.108931.
- American Diabetes Association(ADA): Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021; 44(Suppl 1): S111-24.
- Buse JB, et al: 2019 Update to: Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020; 43(2): 487-493.
- Davies MJ, et al: Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018;41(12): 2669-2701.
- Lehmann R, et al: Swiss Recommendations of the Society for Endocrinology and Diabetes (SGED/SSED) for the Treatment of Type 2 Diabetes, 2020, www.sgedssed.ch/fileadmin/user_upload/6_Diabetologie/61_Empfehlungen_Facharzt/2020_Swiss_Recomm_Medis_EN_def.pdf (last accessed Nov. 24, 2021).
- Drug Information, www.swissmedicinfo.ch, (last accessed Nov. 24, 2021).
- German Diabetes Society (DDG), www.deutsche-diabetes-gesellschaft.de/fileadmin/user_upload/Stellungnahme_Semaglutid_Fusion_210219_final.pdf (last accessed Nov. 24, 2021).
- Marso SP, et al: Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016; 375: 1834-1844.
- Pratley RE, et al. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol 2018; 6: 275-286.
- Rodbard HW, et al: Semaglutide added to basal insulin in type 2 diabetes (SUSTAIN 5): a randomized, controlled trial. J Clin Endocrinol Metab 2018; 103: 2291-2301.
- Novo Nordisk Inc. Ozempic Prescribing Information, 2020 www.accessdata.fda.gov/drugsatfda_docs/label/2017/209637lbl.pdf (last accessed Nov. 24, 2021).
- Aroda VR, et al: Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol 2017; 5: 355-366.
- Bradley C: Diabetes Treatment Satisfaction Questionnaire (DTSQ), 1994, in C Bradley ed, Handbook of Psychology and Diabetes: a guide to psychological measurement in diabetes research and practice. Chur, Switzerland, Harwood Academic Publishers, 111-132.
HAUSARZT PRAXIS 2021; 16(12): 34-35