As one of the most important treatment options for malignant diseases, chemotherapy remains the focus of many therapeutic regimens. However, cisplatin-based treatment in particular is considered highly emetogenic. Nausea and vomiting can be the result. Patients’ quality of life should then be improved by effective control of side effects.
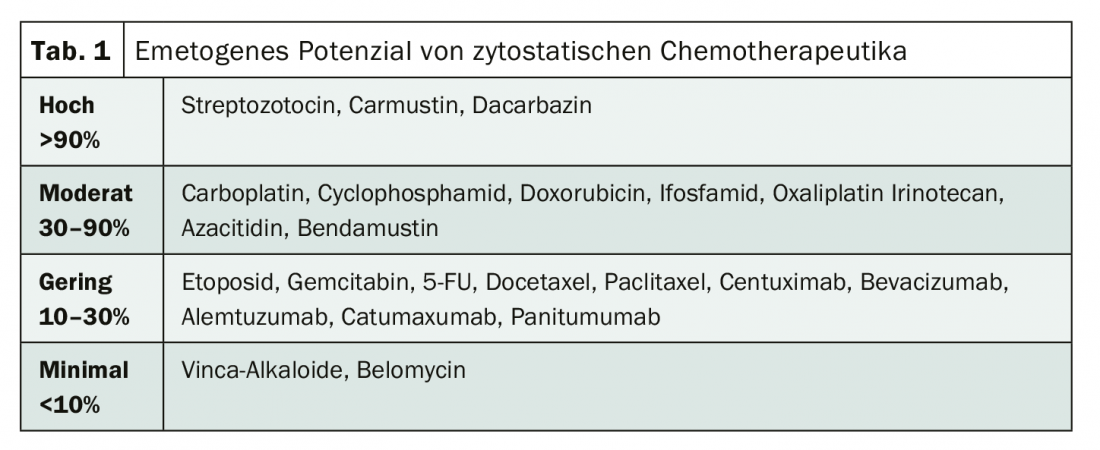
Chemotherapy plays a major role in the treatment of malignant diseases. However, the cytostatic drugs used cause chemotherapy-induced side effects such as nausea and vomiting in up to 50% of patients despite guideline-compliant prophylaxis – with serious consequences for the quality of life of those affected [1]. Therefore, concomitant antiemetic treatment is mandatory in patients undergoing highly emetogenic chemotherapy (Table 1) [2]. It can be divided into acute, delayed, and anticipatory forms of chemotherapy-induced nausea and vomiting (CINV) (Tab. 2). While acute CINV episodes, which usually occur within 1 to 2 hours of chemotherapy initiation, are generally preventable since the introduction of 5-HT3 antagonists, delayed CINV still poses a challenge. It does not set in until 24 hours to five days after the start of chemotherapy, when the patient has already left the hospital. Therefore, the problem is often underestimated, but perceived by those affected as the most unpleasant side effect of chemotherapy [3,4]. On this basis, the German S3 guideline “Supportive Therapy in Oncological Patients” recommends defining and initiating the strategy of antiemesis for the acute and delayed phase of nausea and vomiting already before the start of tumor therapy [4].
Focus on combination therapies
In recent years, the combination of NK1- receptor antagonist, 5-HT3 receptor antagonist, and dexamethasone has been established as an effective option. With one capsule before starting chemotherapy, this combination spares over 80% of patients from CINV – both acute and delayed. Since the results have since been confirmed in everyday practice, this procedure is considered standard [5].

The aim of another combination is to extend this success even further: the atypical neuroleptic with antiemetic properties olanzapine was administered in addition to standard prophylaxis. It was shown that the additional administration of 10 mg/d day 1-4 may well show significant benefits. Over the entire period, 37% of patients did not suffer from nausea. For the triple combination, it was 25% [6]. However, a marked increase in sleepiness appeared. Similar results were obtained in another study in which olanzapine was administered at a dosage of 5 mg on days 1-4 as an adjunct to standard antiemesis. Acute nausea was prevented in 98% vs. 89%, and delayed nausea in 79% vs. 66% [7].
Literature:
- www.krebsinformationsdienst.de/leben/uebelkeit/uebelkeit-index.php (last accessed 03.06.2020).
- Karthaus M: Antiemetic adjunctive therapy: proven and options. Dtsch Arztebl 2016; 113(39).
- Sun CC, et al: Support Care Cancer 2005; 13: 219-227.
- www.awmf.org/uploads/tx_szleitlinien/032-054OLl_S3_Supportiv_2019-11.pdf (last accessed on 03.06.2020)
- Karthaus M, et al: Blood 2018; 132:4844.
- https://deutsch.medscape.com/artikelansicht/4905125#vp_1 (last accessed on 03.06.2020)
- www.dgho.de/aktuelles/news/newsarchiv/2019/download-news-2019/asco-2019-antiemese.pdf (last accessed on 03.06.2020)
InFo ONCOLOGY & HEMATOLOGY 2020; 8(3): 33.