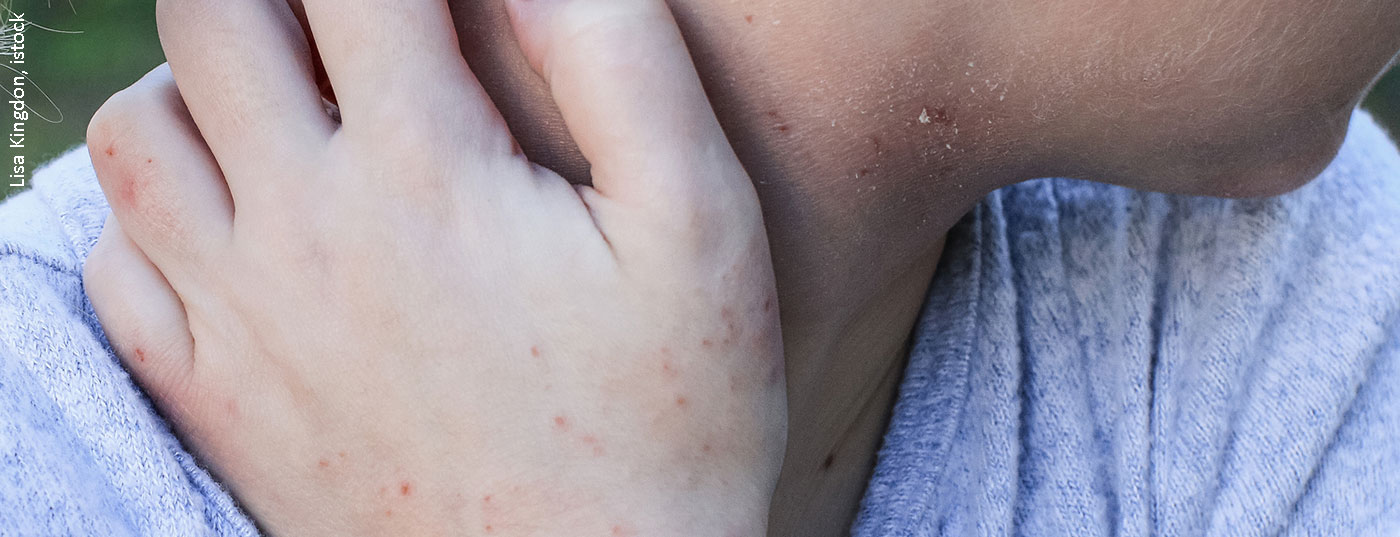
Calcineurin inhibitors can be used in atopic dermatitis as monotherapy or combined with topical corticosteroids. Tacrolimus and pimecrolimus do not cause skin atrophy even with long-term use. Which treatment regimen promises the best efficacy-safety profile and what other current evidence is available is revealed in this article.
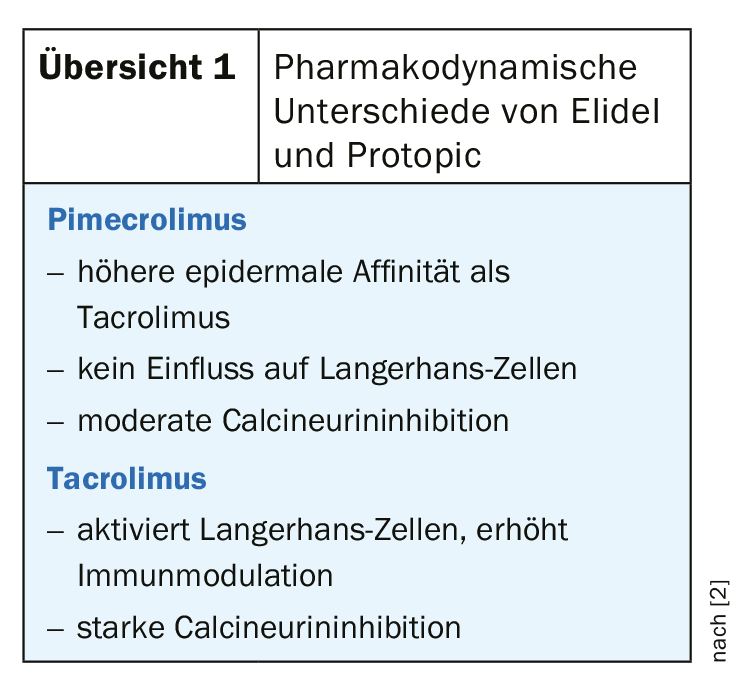
Regarding the efficacy of topical calcineurin inhibitors (TCI) for the indication of atopic dermatitis, there is a relatively large evidence base with over 20 RCTs [1]. At this year’s EADV Congress, Uffe Nygaard, MD, PhD, University Hospital Aarhus reported on the current state of knowledge. Elidel (pimecrolimus 1%) and Protopic (tacrolimus 0.03%; 0.1%) are the calcineurin inhibitors approved in the EU since 2002 for the indication of atopic dermatitis [2] (Overview 1).
Tacrolimus 0.1% proved superior to low-potency topical corticosteroids, pimecrolimus 1% and tacrolimus 0.03% [3]. At the 0.1% concentration, tacrolimus had comparable efficacy to cortisone externals of moderate to strong potency [4], and tacrolimus 0.03% achieved better results than mild steroids and pimecrolimus [5]. In pediatric patients with atopic dermatitis, the efficacy of tacrolimus of both 0.03% and 0.1% concentrations was comparable, with no statistically significant differences [6]. The speaker therefore recommends using the 0.03% formulation [2].
The intensity of burning and itching at the application site correlates positively with the severity of atopic eczema. It is important to know about this connection, Dr. Nygaard, MD, emphasized. It has been shown that in the case of tacrolimus, these undesirable side effects usually disappear within the first week of treatment. The prevalence rates of burning/pain/irritation ranged from 1.6% to 26.7% in a comparative study in the pimecrolimus condition; in the placebo condition, this rate was 1-22.2%, the speaker explained. A study published in 2017 demonstrated that transepidermal water loss-a component of impaired skin barrier function-is associated with an increased risk of burning and pruritus at the application site [7].
Good response in children
According to a systematic review, there is more empirical evidence regarding the long-term safety of TCI in children with atopic dermatitis than regarding the use of topical corticosteroids; TCI also did not cause skin atrophy with long-term use [8]. Therefore, the authors recommend using mainly TCI as standard therapy in pediatric patients and using steroids only in cases of acute exacerbations [8]. The initial side effects at the application site (itching, burning) tend to be less pronounced in children than in adults, the speaker emphasized. Furthermore, he pointed out that the use of TCI in pregnancy is safe and should be preferred to cortisone preparations [2].
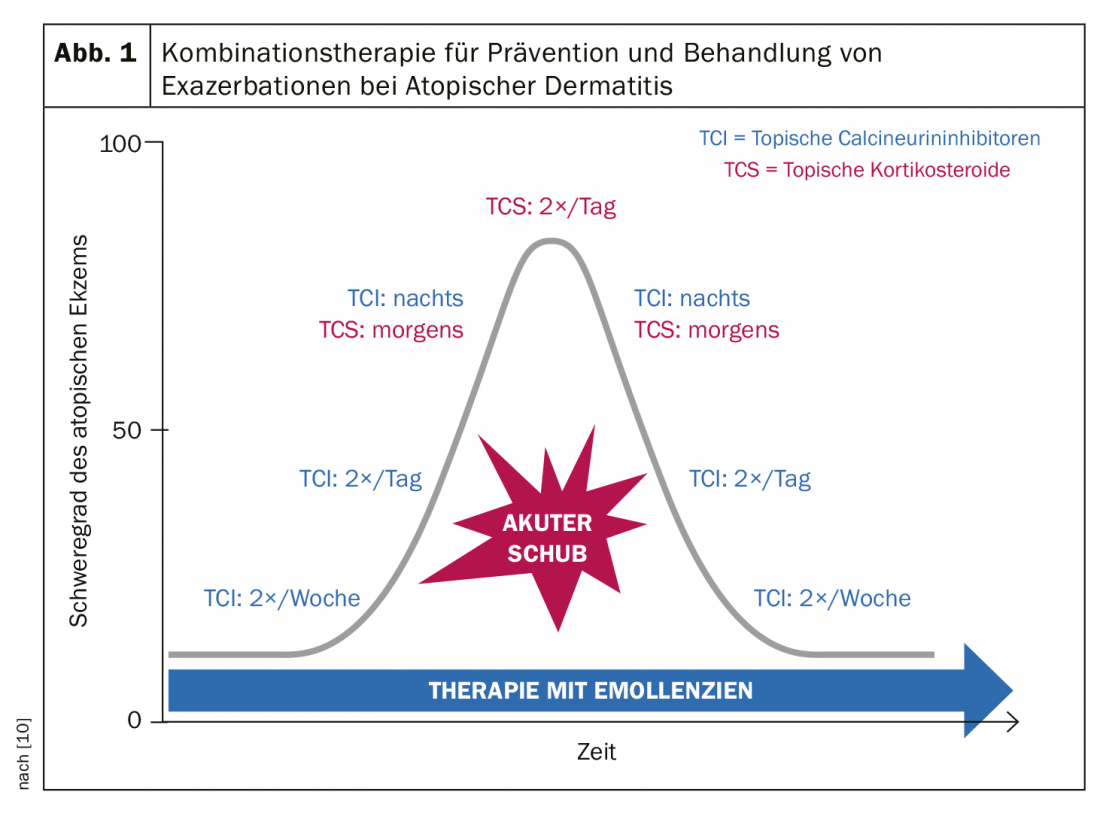
Combined treatment regimen for acute relapses
A recent treatment recommendation by the British Association of Dermatologists recommends TCI as standard therapy, alternating with corticosteroids in case of acute exacerbations [10]. In cases of acute severe eczema flare-ups, TCIs are not strong enough, the speaker explained [2]. The following treatment regimen has been shown to be effective in reducing acute eczematous flare-ups in clinical trials. [10] (Fig. 1): In exacerbations, twice-daily steroid use is recommended during the most acute phase of eczematous flare-ups. With regard to the potency of cortisone preparations, not only the preparation but also the affected body part and personal factors play a role. After symptom relief has occurred, switching to TCI at night in combination with corticosteroids in the morning can be made. With further symptom reduction, the use of cortisone externals can be limited to every other day. TCI should then be applied twice daily, later the frequency can be reduced to once daily. Once the acute episode has completely subsided, the use of TCI can be gradually reduced, initially to every other day and later to twice weekly.
Dr. Nygaard concluded his presentation by pointing out that there are other indications besides atopic dermatitis with high quality evidence of efficacy of calcineurin inhibitors (off label): besides seborrheic dermatitis, this concerns for example psoriasis of the face or the elbows/knees and genitals as well as vitiligo, chronic hand dermatitis, contact dermatitis and oral lichen planus [2].
No empirical evidence of carcinogenicity
In the period 2003 to 2005, there was a decline in the use of TCI in connection with reports of possible carcinogenic effects. However, the conclusion of subsequent studies was that neither for lymphoma nor for other cancers (including non-melanocytic tumors) could the suspicion be corroborated by empirical evidence and that the risk can be assumed to be comparable to the general population [9]. The speaker emphasized that there is no scientific evidence of increased cancer risk with adequate use of TCI.
Source: EADV, Madrid
Literature:
- Martins JC, et al: Topical tacrolimus for atopic dermatitis. Cochrane Database of Systematic Reviews. https://doi.org/10.1002/14651858.CD009864.pub2
- Nygaard U: Calcineurin inhibitors: fears, facts and possibilities. Topical and Systemic Therapies, slide presentation, Uffe Nygaard, MD, PhD, EADV Congress, Madrid, Oct. 12, 2019.
- Chia BKY, Tey HL: Systematic review on the efficacy, safety, and cost-effectiveness of topical calcineurin inhibitors in atopic dermatitis. Dermatitis 2015; 26(3): 122-132.
- Svensson A, et al: A systematic review of tacrolimus ointment compared with corticosteroids in the treatment of atopic dermatitis. Curr Med Res Opin 2011; 27(7): 1395-1406.
- Bieber T, et al: Efficacy and safety of methylprednisolone aceponate ointment 0.1% compared to tacrolimus 0.03% in children and adolescents with an acute flare of severe atopic dermatitis. Allergy 2007; 62(2): 184-189.
- Yan J, et al: Meta-analysis of tacrolimus ointment for atopic dermatitis in pediatric patients. Pediatr Dermatol 2008; 25(1): 117-120.
- Seo SR, et al: Disrupted Skin Barrier is Associated with Burning Sensation after Topical Tacrolimus Application in Atopic Dermatitis. Acta Derm Venereol 2017; 97(8): 957-958.
- Siegfried EC, et al: Systematic review of published trials: long-term safety of topical corticosteroids and topical calcineurin inhibitors in pediatric patients with atopic dermatitis. BMC Pediatr 2016; 16: 75.
- Carr WW: Topical Calcineurin Inhibitors for Atopic Dermatitis: Review and Treatment Recommendations. Paediatr Drugs 2013; 15(4): 303-310.
- British Association of Dermatologists: Calcineurin Inhibitors, Patient Information Leaflets, www.bad.org.uk/for-the-public
DERMATOLOGIE PRAXIS 2019; 29(6): 22-23 (published 8/12/19, ahead of print).