Given the wide variety of targeted treatment options available today, evidence-based criteria are an important toolkit. International experts spoke on this topic at the SGDV Annual Meeting.
While treatment options were once limited to steroids and topical topicals, several conventional systemic therapies are now available and the market approval of biologics have revolutionized treatment options. New representatives of these immunomodulatory substances are available on the market almost every year. According to current pathogenetic understanding, psoriasis is considered a systemic disease in which not only the skin is affected. As we know today, it is a genetically determined dysregulation of the immune system. Comorbidities are common – in addition to psoriatic arthritis, bowel disease, obesity, diabetes, and cardiovascular disease, the prevalence of mental disorders is also increased.
“Targeted therapies” and immunological correlations.
As a basis for the most adequate therapy possible, it is important to understand the immunological patterns, the speaker emphasized [1]. First, increased proliferation of keratinocytes clinically leads to acanthosis and parakeratosis. At the immune cell level, the innate immune system is activated; antigen-presenting cells from progenitor cells generate TH17 cells, which produce inflammation-mediating messengers. On the other hand, TH1 cells are involved, which mainly produce interferon-gamma, resulting in the destruction of the keratinocytes of the basal layer in particular. This manifests clinically in the lichenoid interface pattern. A common feature of these two programs is the p40 subunit of the cytokines IL12 and IL23. Ustekinumab, an IL12/IL23 inhibitor, binds to this p40 subunit of the cytokines IL12 and IL23. The new IL23 inhibitors (risankizumab, tildrakizumab, guselkumab) block the p19 subunit of IL-23. Along with the IL17 antagonists (brodalumab, ixekizumab, secukinumab) and the TNFα blockers (adalimumab, infliximab, etanercept), these agents are among the very effective psoriasis therapeutics available today. A large proportion of patients respond very well to these modern biologics therapies. Questions that arise in connection with the high therapy costs: Which patients receive this well effective but expensive treatment and in which phase of the disease?
Biologics: new generation of highly potent active substances
The introduction of biologics has significantly expanded the therapeutic spectrum in the treatment of psoriasis. Biologics are antibodies produced using recombinant DNA technology that specifically target the function of cytokines, which act as mediators of inflammation. Biologics act quickly and effectively. These are immunomodulators that specifically intervene in the immune system. Since they are protein molecules that would break down in the stomach, they cannot be taken in tablet form, but must be injected subcutaneously or administered by infusion. Currently, the cost of this class of drugs is significantly higher compared to conventional systemic therapies or local treatment, which is related to the technically demanding manufacturing process, which is more costly than for chemically synthesized drugs. A pragmatic criterion for an indication of biologics therapy is the presence of therapy resistance with regard to local treatment and conventional system therapies as well as psoriasis area infestation of more than 10 percent of the skin (PASI>10) or a severe restriction of the quality of life (e.g. with infestation on the palms of the hands, soles of the feet, face or genital area).
Mild severity (PASI <10) is usually treated with local therapy. In addition to steroidal and non-steroidal topical externals, light therapy is also used relatively frequently in Switzerland. In cases in which these treatment methods are not successful and in cases of moderate to severe symptoms, systemic therapies are indicated. Classic conventional systemic therapies for psoriasis include methotrexate (MTX), ciclosporin, fumaric esters, and sometimes acitretin. For all treatment options, the efficacy and tolerability of the ongoing therapy must be monitored regularly and adjusted if necessary (e.g., changes in active ingredient or dose).
Two key arguments for early therapy initiation
In addition to objective severity (Psoriasis Area Severity Index, PASI), quality of life limitations (e.g., Dermatology Life Quality Index, DLQI), response to other therapies, and individual risk profile are important criteria in determining whether and how early in the disease course to use expensive biologics. Based on current data and experience in clinical practice, there are also the following two hypotheses that justify the use of biologics in moderate to severe symptom expression and, depending on the individual risk profile (genetic predisposition), at an early stage of the disease course:
- Hypothesis 1: Development of comorbidity can be prevented
- Hypothesis 2: Early initiation of therapy positively influences the course of the disease
Hypothesis 1: Development of comorbidity can be prevented. There is evidence that comorbidity associated with psoriasis (e.g., arthritis, cardiovascular and metabolic disease) can be reduced with the use of biologics. These concomitant diseases are increasingly taken into account today. Screening for common comorbidities is recommended. Effective drug therapy and lifestyle modification are most effective when combined; for example, it has been shown that when overweight psoriasis patients reduce their weight, the drugs work better. Stress reduction can also lead to an improvement in the symptoms.
According to the speaker, one explanatory model for the development of comorbidities is that proinflammatory messenger substances, which diffuse from the skin into other organ systems, play a decisive role in etiological processes. By intervening early in the disease process at the immune cell level, it may be possible to reduce the risk of comorbidities, explains Prof. Eyerich. However, further studies are needed to be able to make clearer statements on this. At present, the data base is still small, which is due, among other things, to the high costs and great effort of long-term epidemiological studies. However, there are smaller studies that have demonstrated treatment effects on cardiovascular comorbidities. In one of these studies, coronary CT angiography had shown a reduction in atherosclerotic plaques after treatment with TNFα inhibitors and IL17 antagonists over the one-year period [3]. This mainly affected early plaques, which were inflammatory active. The results of this study are an indication that the currently available biologics contribute to prophylaxis of comorbidities, and the earlier therapy is started, the more effective this is. Whether or not appearance-free skin equates to abated inflammatory activity is a question that has not yet been fully resolved, which is related.
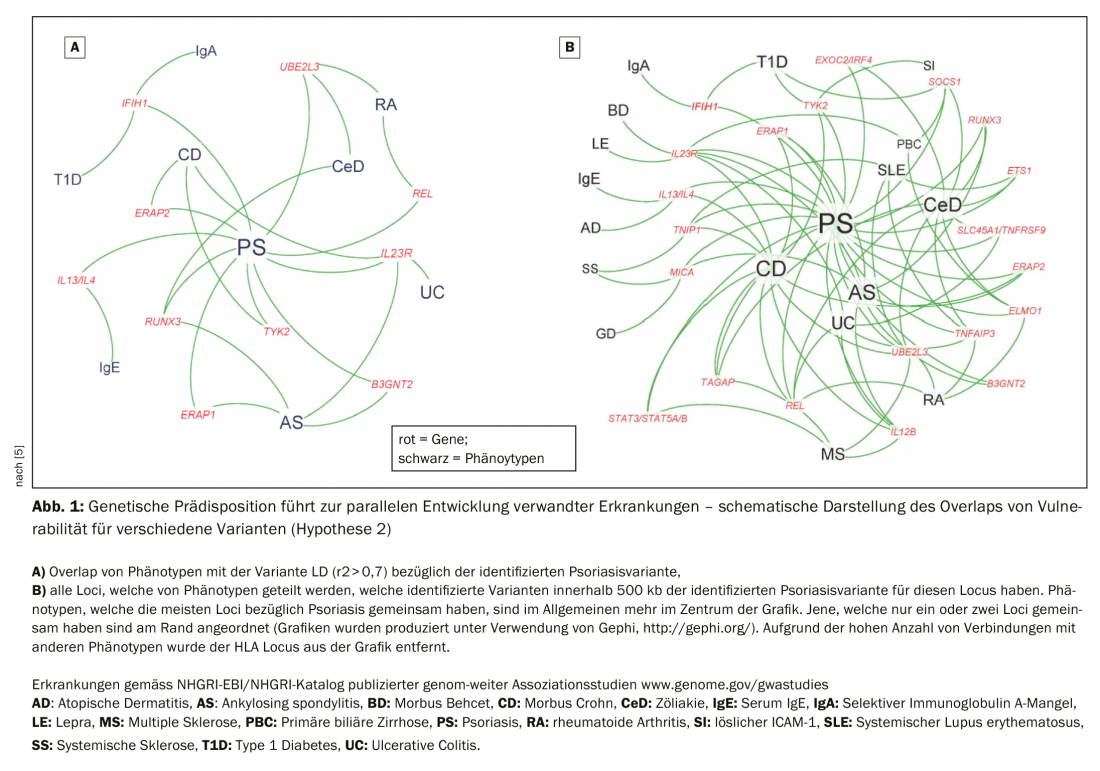
Hypothesis 2: Early initiation of therapy positively influences disease progression. Deciphering the genetic basis of the development of psoriasis is the subject of current research efforts. It is hypothesized that genetic predisposition may lead to the parallel development of related diseases (Fig. 1) . TH17 is therefore not only an immunomodulator for psoriasis, but also has the potential to contribute to the development of rheumatoid arthritis, inflammatory bowel syndrome, Crohn’s disease, MS. Because of gene mutations that are more likely to predispose to a TH17 immune response, one can develop one or the other independently. Recent research findings show that despite lesion-free skin after treatment with immunomodulatory agents (e.g. TNFα), resident memory T cells are detectable in biopsies of the skin [4]. Resident memory T cells form the local immunological memory and probably play a role in recurrence in the same parts of the body, Prof. Eyerich explains. If left untreated, psoriasis leads to a large population of resident memory T cells, which contributes to chronification. Studies are currently underway in Scandinavia and Germany to determine whether early treatment can reduce this immunological memory.
Experience-based assessment as a basis for decision-making
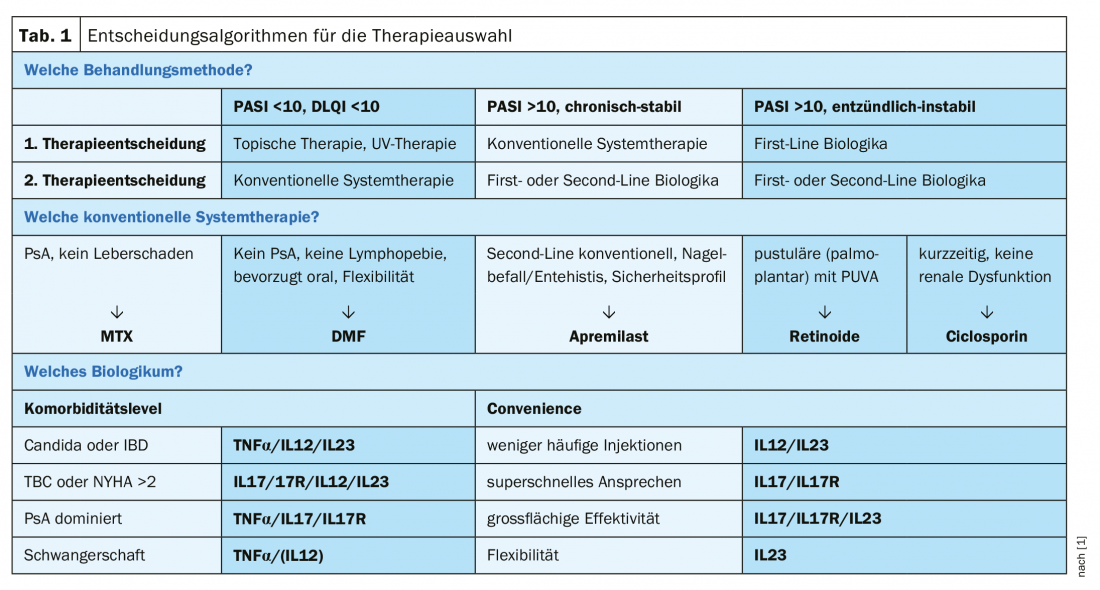
In addition to objective assessment of the degree of expression and decision criteria for therapy selection based on this, clinical experience is also a very important yardstick, the speaker explained. Prof. Eyerich and his team’s approach is that patients with PASI <10 and no criterion supporting systemic therapy receive topical treatment and UV therapy. Patients with PASI >10 and stable symptoms over years receive conventional systemic therapy. First-line biologics, which are currently very expensive, are prescribed to those patients with a highly inflammatory phenotype or those with a lack of response to conventional systemic therapies. Typical clusters of patients are, on the one hand, chronic-stable patients who have the same plaques of similar severity over many years and, on the other hand, those with many small foci characterized by a large dynamic and prone to the formation of inflammatory high-activity pustules. With regard to conventional system therapies, methotrexate (MTX) is frequently used for patients with psoriatic arthritis (PsA) without severe hepatic disease, for which there are also good safety data in long-term use. For patients without PsA and without lymphopenia, dimethyl fumarate (DMF) is a good alternative, he said, although monitoring is important because of the side effect risk of lymphopenia. Nail involvement/enthesitis and a high weighting of the safety profile are criteria that support a second-line indication for apremilast. Unfortunately, ciclosporin is still used in many places; it works well and quickly, but the safety profile is poor and there is often a rebound effect. Therefore, the speaker is critical of the use of ciclosporin in psoriasis treatment. Retinoids are rarely used, usually in patients with pustular forms (e.g., palmoplantar), where topical treatment could be combined with PUVA therapy. With regard to costs, there is a tendency towards a reduction in price differences, but currently therapies with biologics in Switzerland and in Germany are still massively
more expensive than conventional system therapies.
Which biologic in which case?
There are various objective and subjective criteria that can be taken into account when selecting a therapy (Tab. 1) . Taking comorbidity into account is an important factor. For example, IL17 antagonists tend to be contraindicated if someone suffers from Candida or inflammatory bowel disease, explains the speaker [1]. History of tuberculosis or severe heart failure are contraindications regarding TNFα inhibitors. If it is mainly PsA, IL17 antagonists or TNFα inhibitors are preferred according to current data. In the presence of pregnancy, TNFα antagonists are safe, as most data are available. In addition to the data situation, compatibility with lifestyle could also play a role. If it is important to patients that only a few injections are required (e.g., frequent business travelers), IL12 or IL23 would be the therapy of choice. In rare cases of patients for whom a rapid response is important, the speaker recommends IL17 antagonists. In very severe cases, he advises IL17 or IL23 antibodies.
Source: SGDV, Basel
Literature:
- Eyerich K: Psoriasis – Where are we heading? Key Lecture 3, slide presentation, Prof. Dr. med. Kilian Eyerich, TU Munich, SGDV Annual Meeting, Basel 20.09.2019.
- Hawkes JE, Chan TC, Krueger JG: Psoriasis pathogenesis and the development of novel targeted immune therapies. J Allergy Clin Immunol 2017; 140(3): 645-653.
- Elnabawi YA, et al: Coronary artery plaque characteristics and treatment with biologic therapy in severe psoriasis: results from a prospective observational study. Cardiovasc Res. 2019 Mar 15;115(4):721-728. doi: 10.1093/cvr/cvz009.
- Matos TR, et al: Clinically resolved psoriatic lesions contain psoriasis-specific IL-17-producing αβ T cell clones. Clin Invest 2017; 127(11): 4031-4041. doi: 10.1172/JCI93396. Epub 2017 Sep 25.
- Tsoi LC: Identification of 15 new psoriasis susceptibility loci highlights the role of innate immunity. Nat Genet 2012; 44(12): 1341-1348. doi: 10.1038/ng.2467. Epub 2012 Nov 11.
DERMATOLOGIE PRAXIS 2019; 29(5): 32-34 (published 10/10/19, ahead of print).