Recently published data from the multicenter LIBERATE study confirm the favorable benefit-risk profile of apremilast with long-term use. This is a significant advance for the treatment options of this patient population. This is because effective and well-tolerated long-term therapy is essential, especially for moderate and severe courses.
Positive long-term effects have been replicated in several evaluation studies published in 2017 and 2018. The phosphodiesterase 4 (PDE4) inhibitor, which has been approved since 2015, therefore represents a very interesting therapy option for patients who require long-term use, among others.
Favorable benefit-risk profile
Crowley et al. [1] reported in 2017 a positive outcome with the use of the oral PDE4 inhibitor apremilast over a period of up to three years. This represents a replication and extension of the findings of the phase III multicenter ESTEEM 1 and ESTEEM 2 studies, which demonstrated the efficacy and safety of apremilast over a 52-week period [2,3].
This was followed in 2018 by a further replication of the positive long-term effects. The LIBERATE study [4] demonstrated the efficacy and safety of apremilast in biologic-naïve patients with moderate to severe psoriasis over a 104-week period (n=226).
There were improvements in skin, scalp, nails, pruritus as well as quality of life. Apremilast also proved effective and safe 104 weeks after baseline in patients who switched from etanercept to apremilast. 50.0-59%–59,2% of all study participants achieved a ScPGA (Scalp Psoriasis Physician’s Global Assessment) of 0 (lesion-free) or 1 (nearly lesion-free). The mean reduction in pruritus according to the VAS (visual analog scale) was -24.4 to -32.3. The mean change in NAPSI (Nail Psoriasis Severity Index) since baseline was -48.1% to -51.1%. A Dermatology Life Quality Index (DLQI) score ≤5 was achieved by 66.0-72.5% of participants.
An improvement in the quality of life has a positive influence on therapy adherence, which has a favorable effect on the course of the disease. The incidence of adverse events (diarrhea, nausea, nasopharyngitis, respiratory illness, headache) did not increase with increasing duration of apremilast treatment [4].
Benefit for this patient population
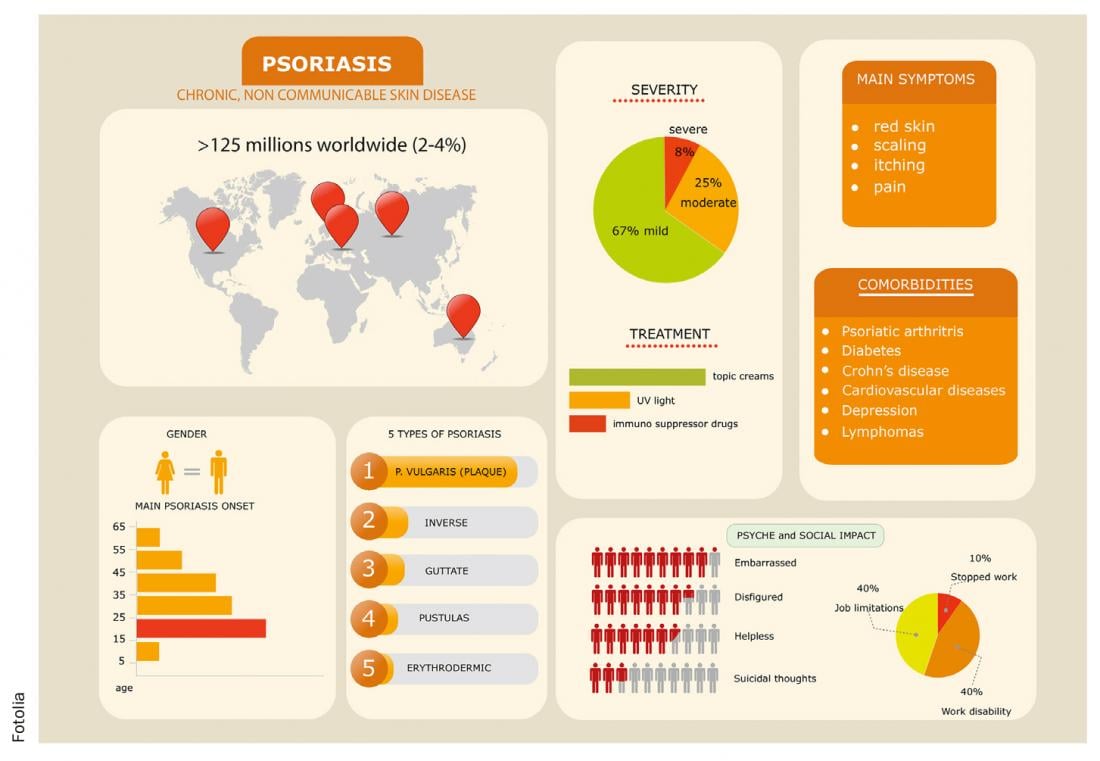
Psoriasis is one of the most common chronic inflammatory diseases of the skin. In the past decades, great progress has been made in the field of research into new therapeutic options. In addition to systemic therapies (methotrexate, cyclosporin A, acitretin), the introduction of monoclonal antibodies led to the achievement of PASI75 (75% improvement in disease symptoms). The exploration of further targets led to the identification of new active substances. In 2015, secukinumab, a monoclonal antibody against IL-17, was approved for moderate to severe psoriasis, showing a timely PASI response and a good efficacy profile in terms of PASI90 and PASI100 (completely symptom-free) for moderate to severe psoriasis.
The phosphodiesterase 4 (PDE4) inhibitor apremilast (Otezla®) has also been approved in Switzerland since 2015 for adults with moderate to severe plaque psoriasis if other systemic therapy is contraindicated, intolerant or refractory. PDE-4 inhibitors reduce inflammatory processes by regulating the concentration of cyclic adenosine monophosphate (cAMP), an important modulator of the immune response. An increase in the cAMP concentration of inflammatory cells by the PDE-4 inhibitor apremilast (Otezla®) leads to a decreased release of TNF-α, IL-17 and IL-23 and an increase in anti-inflammatory IL-10 [5].
Overall, the conclusion regarding treatment goals and adherence is positive.
Literature:
- Crowley J, et al: Long-term safety and tolerability of apremilast in patients with psoriasis: Pooled safety analysis for ≥156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol 2017; 77(2):310-317.e1. doi: 10.1016/j.jaad.2017.01.052. Epub 2017 Apr 14.
- Papp K, et al: Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM 1]). J Am Acad Dermatol 2015; 73: 37-49.
- Paul C, et al: Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate to severe plaque psoriasis over 52 weeks: a phase III, randomized, controlled trial (ESTEEM 2). Br J Dermatol.2015; 173: 1387-1399.
- Reich K, et al: Safety and efficacy of apremilast through 104 weeks in patients with moderate to severe psoriasis who continued on apremilast or switched from etanercept treatment: findings from the LIBERATE study. J Eur Acad Dermatol Venereol. 2018 Mar;32(3):397-402. doi: 10.1111/jdv.14738.
- Wick-Urban B: Antipsoriatics. A comparison of new therapies. Pharmaceutical Journal. Issue 09/2017, February 27, 2017, www.pharmazeutische-zeitung.de/ausgabe-092017/neue-therapien-im-vergleich/, last accessed May 09, 2019.
- Kavanaugh A et al: 5-Year Efficacy and Safty of Apremilast Treatment in Subjects With Psoriatic Arthritis: Pooled Analysis of the PALACE Studies. Poster THU0294 presented at the EULAR; June 13-16 2018 in Amsterdam, Netherlands.
DERMATOLOGIE PRAXIS 2019; 29(4): 48