CGRP has potent vasodilatory properties and plays a central role in pain initiation as well as neurogenic inflammation. This knowledge gave rise to a completely new class of substances that, in the context of previous options, represents a quantum leap in migraine prophylaxis.
Triptans, which are used in the treatment of migraine, also inhibit the release of CGRP, among other things, by acting as agonists at 5-HT1 receptors. Migraine is known to be characterized not only by headaches. Patients are often nauseous, lack appetite, have visual disturbances, and are hypersensitive to light and sound. These symptoms are also associated with a significant impairment of quality of life. This makes it all the more important to be able to offer these chronic patients a well-tolerated prophylaxis with few side effects, said Prof. Dr. med. Uwe Reuter of the Charité Berlin at the German Pain Congress. Furthermore, the goal of drug prophylaxis is not only to reduce the frequency, severity, and duration of migraine attacks, but also to reduce triptan use and other analgesic use and to prevent drug-induced persistent headache, which can occur as analgesic use increases.
Reduce chronic and episodic migraine
The criteria for chronic migraine are met if a patient suffers from headache on at least 15 days per month and reports migraine-type headache on at least eight of those days. In episodic migraine, on the other hand, sufferers experience migraines at varying intervals of up to 14 days per month.
Three CGRP antibodies were shown in studies to be more effective than placebo and to result in a significant decrease in migraine days. All three substances lead to a significant therapeutic effect already in the first four weeks of therapy and are also well tolerated. They have a side effect profile that is at placebo level with the exception of injection site reactions. Specifically, these are the CGRP receptor-binding antibody erenumab, which is already approved for the prophylaxis of migraine in adults on the European market, the antibody galcanezumab, and the monoclonal antibody fremanezumab, which can be administered quarterly or monthly. Two of the compounds, galcanezumab and fremanezumab, directly target CGRP, while erenumab targets and inhibits the CGRP receptor.
A direct comparison of efficacy between the three substances is not available; studies are hardly comparable due to different study designs and duration. All three monoclonal antibodies are more effective than placebo and, in an indirect comparison, as effective as previously used migraine prophylactics.
Migraine prophylaxis to date: the dose makes the poison
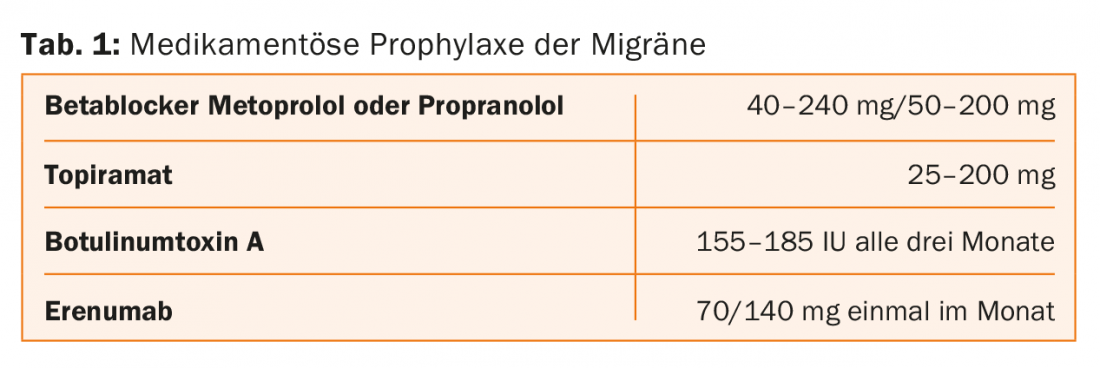
Current prophylactic therapies were originally developed for other indications and are often associated with poor tolerability and/or insufficient efficacy, he said. Beta-blockers show good efficacy in reducing migraine attacks. However, high doses are required, such as 160 mg propranolol. “What patient can tolerate 160 mg of propranolol?” the speaker asked provocatively. Potency disorders, low blood pressure and fatigue occur frequently at this dose. The situation is similar with the anticonvulsant topiramate, which is an alternative to beta blockers in the prevention of migraine. In the case of topiramate, a dose of up to 200 mg per day is necessary. Adverse effects are common at this dose, he said, including paresthesias, drowsiness, insomnia, difficulty concentrating, and memory impairment. The usual dosage prescribed in the outpatient migraine clinic at Charité is 50 mg; at this dosage, the efficacy of topiramate is just above placebo.
Two days less headache per month and triptan reduction
In the prophylaxis of chronic migraine, botulinum toxin A has become established (in Germany). It is injected in a dose of 155 or 185 IU every three to four months in the area of the forehead, temple, occiput, neck and shoulder muscles.
With botulinum toxin A, copain days decrease by eight days per month compared to six days with placebo. So the bottom line benefit is two days per month. With treatment with erenumab, patients with episodic migraine and an average of eight migraine days per month experienced a decrease in studies
by about three days. The monoclonal antibody erenumab also reduces headache days in patients who have not responded to other prophylactics.
Number Needed to Harm note
The choice of a drug in the prophylaxis of migraine depends, on the one hand, on its efficacy and, on the other hand, on the potential side effects, its tolerability, and the likelihood of causing side effects.
“The likelihood of helping a migraine patient (chronic or episodic migraine) with erenumab is very high,” Prof. Reuter said, referring to a study just published in the journal Cephalagia [1]. It is much higher than with the prophylactics used so far. In addition, the Number Needed to Harm, the number of treated patients it takes to produce an adverse event, is at placebo level with erenumab, 13 with topiramate, and 39 with botulinum toxin.
In summary, there is no reason (other than monetary) to withhold the new prophylaxis from migraine patients, Prof. Reuter said.
The drug prophylaxis of migraine is shown in Table 1.
Source: German Pain Congress, October 17-20, 2018, Mannheim (D).
Literature:
- Vo P, et al: Benefit-risk assessment of erenumab and current migraine prophylactic treatments using the likelihood of being helped or harmed. Cephalalgia 2018 Sep 19: 333102418801579.
Further reading:
- Reuter U, et al: Efficacy and tolerability of erenumab in patients with episodic migraine in whom two-to-four previous preventive treatments were unsuccessful: a randomised, double-blind, placebo-controlled, phase 3b study. Lancet 2018 Oct 22. DOI: 10.1016/S0140-6736(18)32534-0 [Epub ahead of print].
InFo NEUROLOGY & PSYCHIATRY 2018; 16(6): 54-55.
HAUSARZT PRAXIS 2018; 13(11): 34-35











