Traces of gadolinium can be detected in tissues after multiple applications using modern chemical methods, even over time, but without clinical relevance or associated sequelae. Nevertheless, restrictions have recently been imposed on some gadolinium preparations within the EU.
Clinically introduced in 1989, gadolinium compounds as contrast agents (Gd-KM) soon began a triumphant march thanks to the potential of magnetic resonance imaging (MRI) but also thanks to their good tolerability, especially compared to CT contrast agents. Popular at the beginning to somewhat shorten the long examination times, gadolinium compounds have been used more and more widely and also in increasing doses in T1 contrast weighting on MRI. Gadolinium shortens and catalyzes the T1 relaxation time of protons buzzing around the atom in the immediate vicinity, thus increasing the so-called T1 signal. This allows for better detection and characterization of certain lesions. Initially used only for CNS questions to facilitate the detection of pathologies, their characterization and the tracking of a treatment response, the field expanded to clinical questions practically in the whole body [1,2]. In doing so, it was believed that the gadolinium-containing MR KM would be rapidly and completely excreted by the body.
Indications for the use of the Gd-KM
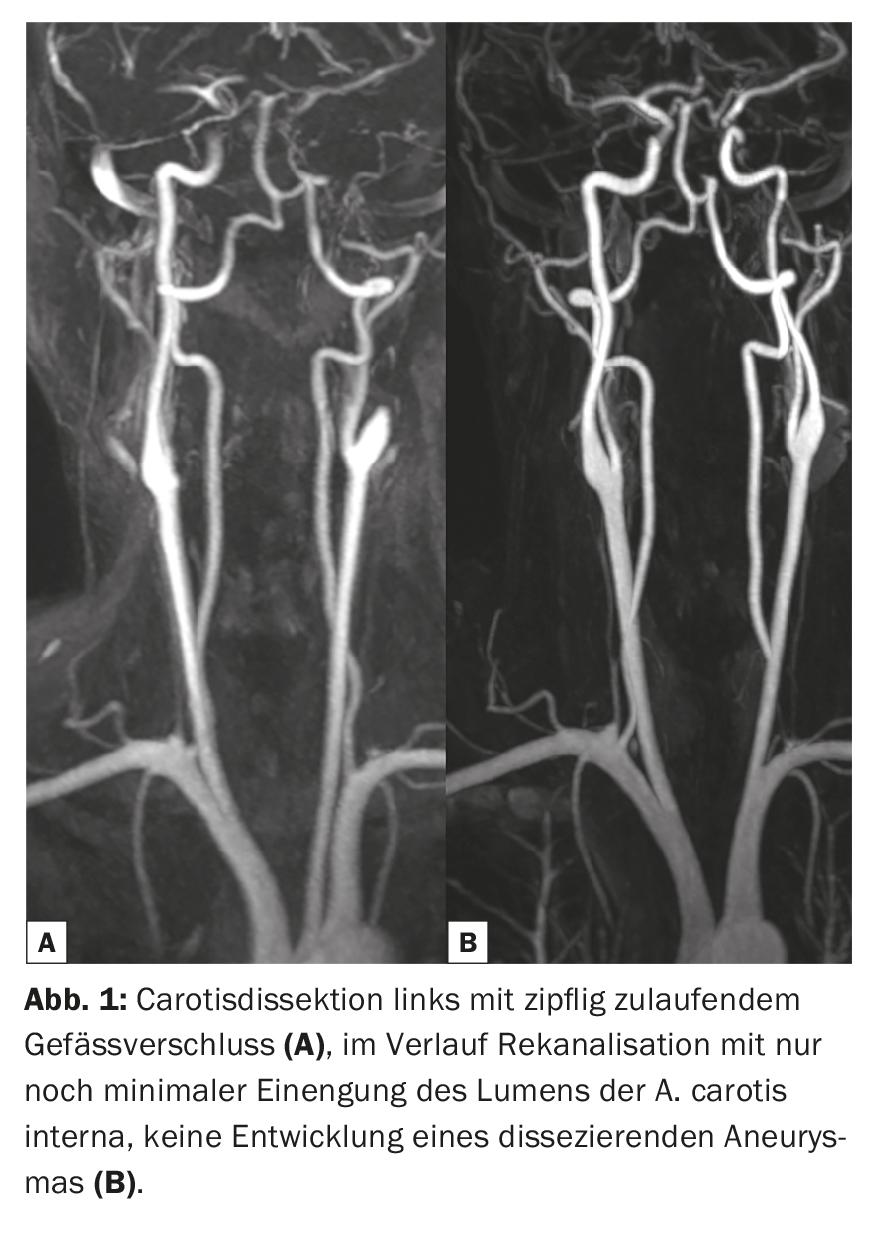
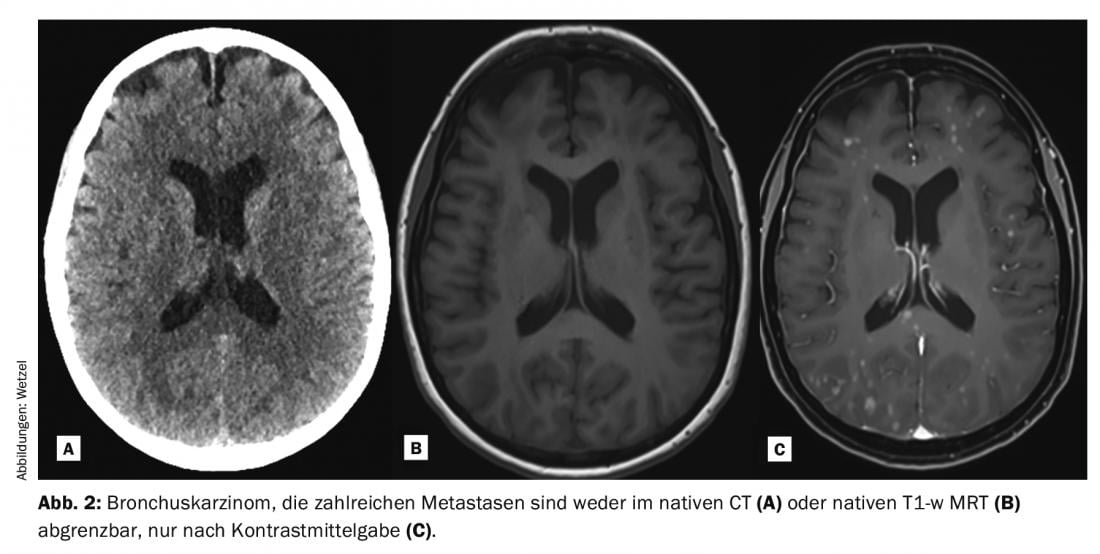
In clinical practice, T1-weighted Gd-KM enhanced pulse sequences are often measured and converted to images after the native examinations. This occurs throughout the body depending on the issue, although individual pathologies are more likely to rely on the use of Gd-KM than others. Thanks to the increased T1 signal of protons (signal-rich) in the contrasted lesions after Gd-KM, they can often be better detected and delineated from the surrounding tissue. The prerequisite for the signal to change is the T1 weighting, the correct dose and also the specific kinetic properties, especially in the CNS area with the passage through the disturbed blood-brain barrier. Typical indications for which Gd-KM is administered to evaluate the brain parenchyma are the diagnosis of tumor diseases, multiple sclerosis, inflammatory diseases, and vascular pathologies (Fig. 1-3). Despite enormous technical progress, there are numerous pathologies and lesions that even today can only be visualized thanks to the administration of contrast media. Thus, Gd-KM are also frequently used simply to rule out pathology [3]. Another important indication for Gd-KM is the classic imaging of vascular flow path areas, mostly of the arterial region. Radiation-free imaging of vascular stenoses, obstructions or even bypasses was first made possible with MRI and Gd-KM.
MR perfusion analysis represents another recent area in the application of KM. Tumors often result in increased contrast between the tumor and surrounding tissue due to increased vascularization, termed neoangiogenesis, and more pronounced vascular leakage, termed increased “vascular permeability” (leakage). The flooding of the contrast medium into tumor-affected foci, inflammations, or infections often occurs with characteristic inflow and outflow curves, and often makes it possible to interpret a clinical question that would remain unclear without contrast medium.
Perfusion characterization of suspect foci is now increasingly performed using pharmacokinetic models. Apart from improved detection, contrast correlating with blood flow can also better characterize the progression of a tumor focus and its aggressiveness. The typical perfusion patterns can be used to break down further endpoints very specifically using color-coded images and encode them pixel by pixel in a site-specific manner.
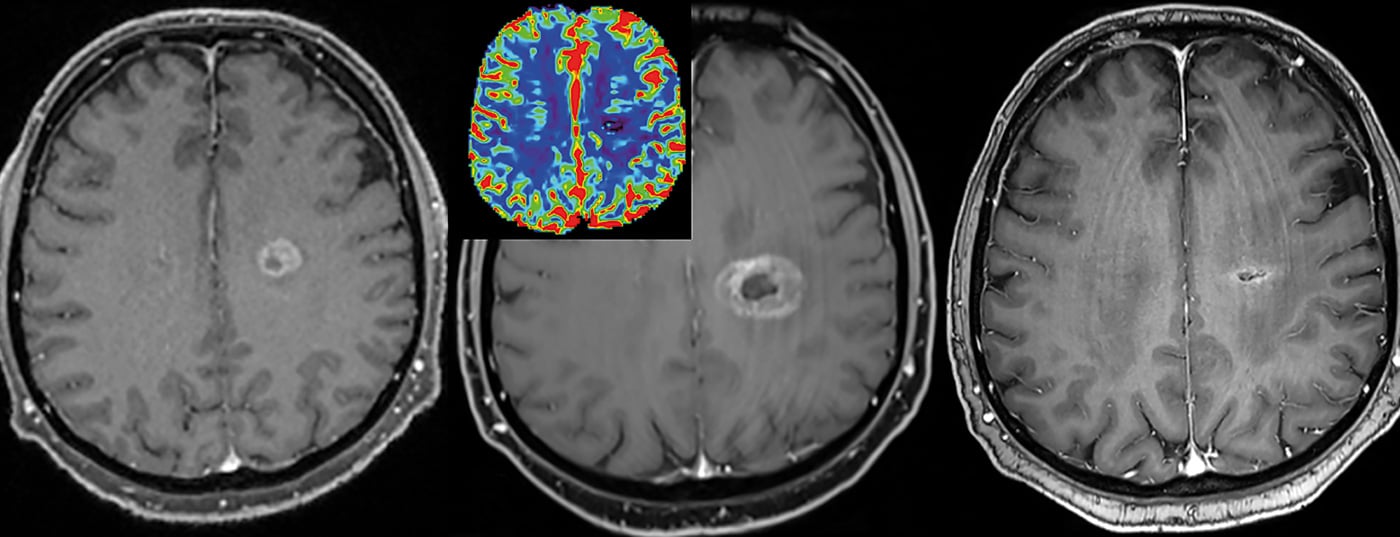
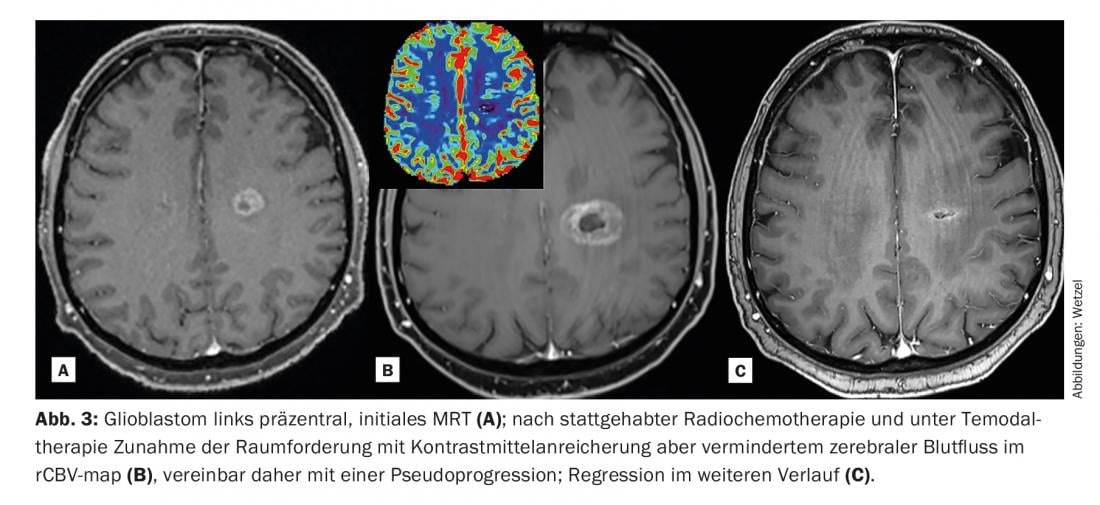
This better captures and differentiates the heterogeneity of a tumor [2]. This in turn has consequences for further treatment and for therapy monitoring (especially also for radiotherapy). Lesions can thus be tracked relatively well over time, and in particular characterized and monitored by means of quantitative corner points.
Properties of the Gd-KM
Gadolinium is used thanks to its “super-single” activity – it is the only element in solution that has seven individual electron spins (singles) that interact ideally with water protons in tissue. Thus, gadolinium solutions exhibit a pronounced paramagnetic property. The energy exchange between the electrons of the gadolinium on its outer valence shells and the protons excited by the MR signal is what enables the contrast enhancement in the T1 image in the first place. Shorter T1 times thus lead to more signal and also selective contrast patterns wherever the contrast agent spreads. Even though there is simplistic talk of gadolinium as an MR contrast agent, it must be emphasized that actually gadolinium complexes are used clinically as MR contrast agents (=Gd-KM). They consist of the central atom (Gd3+) and the chelators or ligands that surround it. The chelators or ligands bind the gadolinium extremely tightly, forming a complex bond. The active ingredients are available dissolved in the form of standardized gadolinium solutions (Gd-KM), which can usually be injected i.v. or diluted intraarticularly. They have lower concentrations and thus lower systemic exposure compared to CT contrast agents (approximately 0.01 moles with Gd-KM versus 0.1 moles with X-ray contrast agents).
The gadolinium complexes are highly water soluble, distribute vascularly and interstitially with a distribution half-life of approximately two to five minutes, and if nephrotropic are excreted renally unchanged by glomerular filtration with an elimination half-life of approximately 90 min. Small amounts of Gd-KM pass the placental barrier or can also be detected in breast milk. In liver-specific MRKM, receptor-associated kinetics with selective hepatocyte uptake and excretion into the bile ducts occur alongside parallel renal elimination. The hepatic receptivity results from anion receptor specific side groups that were attached to the ligands.
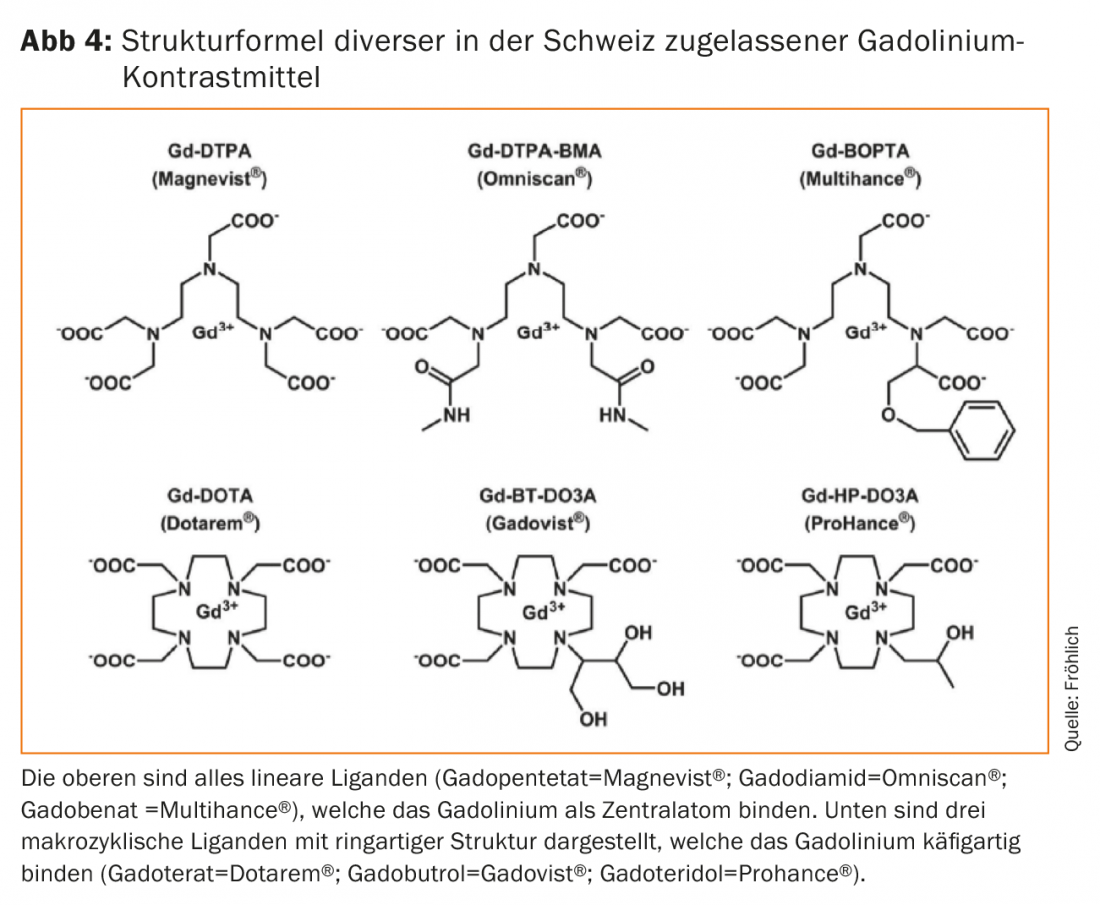
The ligands that thermodynamically bind the gadolinium are either present in a cyclic, ring-like structure as so-called macrocycles or in a chain-like form as linear ligands (Fig. 4) . The macrocycles prove to be significantly more stable thermodynamically, although the clinical significance of this property was only recognized later. If gadolinium dissociates from the complex bond – i.e. frees itself from the complex bond, so to speak – it precipitates as hydroxide or phosphate in the plasma, accumulates in the liver, spleen as well as in macrophages and interacts as a calcium blocker with numerous calcium-dependent metabolic processes (blood coagulation, mitochondrial respiration, bone turnover). The assessment of toxicity effects is further complicated by interactions with endogenous electrolytes such as zinc, iron, copper or even calcium. Thus, the goal is clear, a release of gadolinium from the complex binding must be prevented by all means [5].
Nephrogenic systemic fibrosis [6]
Gadolinium experienced its first setback in 2006, when the young nephrologist Thomas Grobner of the Landesklinikum Wiener Neustadt first observed a link between gadolinium administration in five of his dialysis patients and their marked worsening of a previously unexplained mysterious skin disease (nephrogenic fibrotic dermopathy) [7]. The enigmatic disease had first appeared in clusters in individual American cities in 1997 and was not published in The Lancet until 2000 [8]. Only three years later, the disease was renamed “Nephrogenic Systemic Fibrosis (NSF)” due to lethal systemic fibrosis with corresponding internal scarring (fibrocyte activation) in certain patients [9]. It was not until 2011 that the disease was clearly defined and delineated both histopathologically and clinically [10]. Numerous case series followed, steadily increasing the fear of NSF as well as speculation about its trigger. Hypotheses ranged from a dialysis problem (although not all affected were dialysis patients) to infectious agents. Even the registry of over 300 cases run by Cowper at Yale could not provide a plausible explanation for NSF. Only Grobner and a little later the Copenhagen group around Henrik Thomsen partially clarified the mystery. Apparently, Gd-KM circulated in renal insufficients until it dissociated and was released in tissues in individual cases, especially in more unstable complex compounds. In certain patients this resulted in a foreign body-like, very painful intoxication with a marked mobilization and proliferation of so-called tissue fibrocytes, on detection of CD34 positive cells, increased procollagen type I amounts, more often associated with complications if postoperative or with an inflammatory disposition [11]. Shortly thereafter, contractures and significant muscle impairments occurred peripherally but also sporadically in the trunk with further complications. Despite the use of different therapies, patients could only be treated successfully in a few cases.
The drug authorities, first the American FDA in June 2006 and shortly afterwards Swissmedic, reacted purposefully and extremely quickly. They urged careful consideration of contrast-enhanced MRI in severely renal insufficient patients, use of the lowest possible dose, and attention to clinical alarm symptoms. Later, in February 2007, the European EMA followed with contraindications for two linear unstable gadolinium contrast agents in pre-existing renal insufficiency (eGFR<30ml/min/1.73m2). The precautions defined in 2007 and later in 2011 explicitly highlight the benefits of gadolinium in an indicated MRI examination. To prevent further cases of NSF, patients with impaired renal function and thus longer circulation times were especially better protected. The linear, unstable contrast agents without hepatic excretion could practically no longer be used in renal insufficiency; otherwise, doses were limited as much as possible and, if necessary, macrocyclic gadolinium preparations were used only after careful risk-benefit assessment. These recommendations were based, on the one hand, on epidemiologic data demonstrating higher relative NSF case rates with the linear MRKM gadodiamide, gadoversetamide, and gadopentetate, but also on numerous preclinical studies demonstrating stability differences and consequences. Thus, macrocycles gained high-risk patient status, especially in patients with known preexisting renal insufficiency. Gadoterate, which had been used prospectively in several hundred patients requiring dialysis in France without leading to NSF, was considered relatively safe.
Gadolinium deposits in the brain
Already from 2010 onwards, thanks to the multistage measures, a dose restriction, a greater restraint as well as an increasing switch to macrocyclic MRKM, no new NSF cases occurred, so that a general sigh of relief was felt [12] until the end of 2014 in the journal Radiology, Japanese researchers led by Tomonori Kanda found a correlation between the number of repeated MRI examinations with gadolinium complexes and increased signal intensities in the T1 image, this time in renal healthy subjects. [13]. This mainly affected the dentate nucleus as well as the globus pallidus already on the native images, whereas visible signal changes were mostly described only after five to six examinations as a result of the administration of rather unstable, linear gadolinium preparations. Later, similar phenomena could be measured in other CNS structures [14]. Since similar contrast patterns had also been described from various metal intoxications, multiple sclerosis or even after irradiation, the specificity of the effect was questioned. Then, a little later in 2015, McDonald was able to demonstrate that the elevated signals in the dentate nucleus in his patients were indeed gadolinium deposits [15]. The gadolinium could be chemically detected and localized. It often occurred in the perivascular area, but sometimes in more distal tissue areas. It should be emphasized that the form in which the gadolinium was stored in the tissue was anything but clear. Thus, the question remains whether the gadolinium in free dissociated form was at best bound to phosphates or hydroxides or continued to persist in the tissue in a complex bound form.
Numerous retrospective case series followed, which, similar to Kanda, showed significant signal differences between the unstable, linear complexes and the more stable, macrocyclic gadolinium preparations. Macrocyclic gadolinium preparations did not result in measurable signal changes in most cases (even after multiple repeated use), whereas the more unstable ones virtually always resulted in a signal increase that was clearly measurable on MRI as a function of the number of Gd-KM doses administered. Actually, animal studies from the early 1990s had already shown such differences in the flushing of gadolinium complexes from the various tissues, but it had always been assumed that this was of little practical significance. The new data with apparently also long-term visible signal changes (respectively residues) were worrying, even if no visible disease or pathology could be assigned to these signal changes. Thus, the recently detected T1 signaling change in CNS tissue is quite distinct from the intoxication associated with gadolinium known as NSF. Especially since the long-term effects are unclear, the EMA ordered at the end of 2017 that the distribution of all linear gadolinium compounds that are not liver-specific must be stopped in Europe [16]. Exceptions remain the contrast agents for direct MR arthrography (gadopentetate; Magnevist® 2.0 with 0.002 mol Gd/lit) in addition to the macrocyclic gadoterate (Artirem® with 0.0025 mol Gd/lit). Both are injected directly into various joints at very low doses and concentrations for clinical use. Likewise, the linear, liver-specific Gd-KMs such as gadobenate (Multihance®) or gadoxetate (Primovist®) remain on the market. The macrocyclic MR-KM gadoterate (Dotarem®), gadobutrol (Gadovist®) or gadoteridol (Prohance®) may continue to be used diagnostically at the lowest possible dose thanks to their complex stability. Gadolinium, as a potentially toxic central atom, can be better trapped in entropy and also kinetically thanks to the ring structure of these ligands.
Given the uncertainty regarding the clinical relevance of T1 signaling changes in diverse CNS structures, later also with gadolinium deposition demonstrably in additional tissues (bone, kidney, reticuloendothelial) [17,18], numerous new questions arose around gadolinium. How does it pass through the blood-brain barrier into the two brain areas mentioned above? Is it slowly excreted from there? Can it slowly dissociate and be released there? Can NSF-like consequences occur locally? What about deposits in the rest of the body tissue? How do macrocycles compare to linear KM? Besides the acute observations, are there also long-term consequences that cannot even be properly assessed yet?
Thus, in addition to the acute tolerability profile of gadolinium preparations, which, like most contrast media, can lead to hypersensitivity reactions (allergic, allergy-like) or, at higher doses, to KM-induced nephropathy, questions surrounding the subchronic and chronic tolerability of Gd-KM now gained importance. Ultimately, with longer residence times or even small residues, experts cannot completely rule out some risk of chronic or late-onset intoxication symptoms, even if there is no evidence of this, and despite several hundreds of millions of Gd-KM applications in total.
And more?
Not entirely unexpectedly, because MR imaging involves virtually all pathologies, other partially unexplained adverse drug reactions (ADRs) associated with Gd-KM administration occurred. It was not always clear whether the MR examination or the contrast agent administered was responsible for the symptoms observed. In particular, the two experts Semelka (Durham) and Ramalho were the first to postulate a so-called “gadolinium deposition disease”, which they found in rare cases and without a clear causal relationship in their MR patients even with completely normal renal function [19]. Typical clinical manifestations include a whole range of non-specific persistent headache, bone or joint pain. This is sometimes accompanied by subcutaneous soft tissue proliferation, thickening, peripheral arm and leg pain, which is described as burning or cutting. These effects occurred in some cases after only one application of gadolinium and different periods of time (a few days to months) after MR examination and could not be assigned to other diseases. Sometimes gadolinium could be analyzed in the plasma, urine or even tissue samples of the patients. Some genetic predisposition with increased sensitivity has been discussed. Alternatively, the possible environmental contamination with traces of gadolinium also in wastewater and thus in the food chain increasingly became the focus of interest with demands for better wastewater treatment around hospitals or for MR investigations. Although numerous toxic mechanisms and interactions of gadolinium with endogenous functions have been described, which might play a role especially in the field of cytokine- and chemokine-driven toxicity, calcium channel effects, reactive oxygen radical formers or in the field of MMPS as well as neurotoxicity, a clear causal relationship is missing.
The trace amounts of gadolinium, sometimes still detectable after months to years, in CNS tissue but also in the rest of the organism, with higher amounts after linear Gd complexes lead to the question how such kinetics can be explained. Thanks to an intensive research collaboration between the Department of Forensic Medicine at the University of Zurich and the Department of Radiology at the Cantonal Hospital in Baden, they were one of the first research groups ever to prove clinically that the injected gadolinium can also be detected in small quantities in the cerebrospinal fluid [20]. In more than 60 patients who had undergone MRI and in whom CSF sampling was clinically indicated, the gadolinium content could be quantified by ICP-MS compared to a control group. Rather unexpectedly, all patients exposed to gadoterate showed a corresponding positive measurement with sometimes high differences depending on the patient and also pre-existing disease, whereby the analytical devices used are extremely sensitive. In two control patients, who were also positive for gadolinium in CSF, a previous MRI examination with gadolinium was subsequently demonstrated. Over approximately eight hours after injection, the concentration of gadolinium in the CSF increases slowly but steadily, followed by slow, protracted elimination over very long periods. This results in a much later peak and very slow elimination compared to plasma. Measurements in CSF were made using the very sensitive and precise ICP mass spectroscopy method, which, however, does not allow differentiation with respect to the chemical mode of binding of gadolinium (whether free or tightly complexed). The CSF measurements could be the key to the passage of gadolinium even with an intact blood-brain barrier – probably this occurs via the choroid plexus and, at most, further via the glymphatic circulation – although this is all still speculative. Depending on the stability of the gadolinium complexes, a different dissociation rate, transmetallation and exchange with other electrolytes, metal ions and also phosphates/hydroxides will again be expected in the CSF.
Many questions regarding elimination and redistribution from these distal compartments to specific brain areas, if any, remain unanswered; what form the gadolinium ultimately remains in, whether it is redistributed, and whether it is slowly excreted via the perivascular spaces, if any, need to be clarified. In principle, it must be emphasized that even the experts do not agree on the significance of the above observations. In particular, the significance of numerous, subtoxic effects of the various forms of gadolinium (depending on the binding form and dose) with preclinical or even clinical consequences, remains open. Thus, the induction of necrosis and apoptosis in renal tubule cells, increased cytotoxicity or even further nephrotoxic, hematotoxic, neurotoxic or even inflammatory reactions have been described [21]. While the European authorities have categorically banned the more unstable, linear gadolinium preparations from the market, the U.S. health authorities are generally more cautious, recommending the use of the more stable, macrocyclic Gd preparations in cases of repeated use or even in pediatrics, but certainly allowing the continued clinical use of the more unstable linear complexes. This is with the comment that, to date, no adverse effects have been reported with respect to gadolinium deposition, no histopathological changes have been demonstrated, and numerous unanswered questions remain with respect to kinetics. The Swiss medicines authority Swissmedic has not yet taken a clear position.
As is so often the case in modern medicine, we got carried away somewhat uncritically out of sheer enthusiasm for the new low-radiation imaging technique with pronounced soft-tissue contrast and excellently tolerated Gd-KM. It is now necessary to use gadolinium-containing contrast media in a wellindicated and judicious clinical manner. Using the more stable macrocyclic gadolinium complexes or single inserts of liver-specific MRKM, there is no evidence of any associated sequelae at the present time, despite the trace amounts and possible T1 signal increases in various tissues, even over longer periods of time. On the one hand, it is important not to misinterpret the possible signal increase after sequential administration of several doses of gadolinium on the native images, and on the other hand, to compare the potential benefit of gadolinium administration with the extremely low risk. Patients should be informed of the overriding benefit of gadolinium contrast administration when clinically indicated and to rule out pathology.
Take-Home Messages
- The Gd-KM are usually used i.v. at a dose of 0.1 mmol Gd/kg body weight. Special applications such as liver studies or vascular angiography require lower doses. Direct MR arthrography, in which Gd-KM is injected intra-articularly, requires very low doses of Gd-KM.
- Gd-KM should be used at the lowest possible dose. With repeated administration and corresponding risks, each administration should be carefully weighed and the more stable macrocyclic Gd-KM should be used. In renal insufficient patients with an eGFR <30 ml/min/1.73 m2, a maximum of one single dose of Gd-KM (=0.1 mmol/kg bw) may be administered over seven days.
- Traces of gadolinium can be detected in tissues after multiple applications using modern chemical methods (ICP-MS), even over time, but without clinical relevance or associated sequelae.
- The specific properties of each gadolinium preparation must be considered in clinical practice.
Literature:
- Czeyda-Pommersheim F, Martin DR, Costello JR, Kalb B: Contrast agents for MR imaging. Magn Reson Imaging Clin N Am 2017; 25: 705-711.
- Yuan Jm Chow SK, Yeung DK, King AD: A five-colour color-coded mapping method for DCE-MRI analysis of head and neck tumours. Clinical Radiology 2012; 67: 216-223.
- Roberts TP, Mikulis D: Neuro MR: principles. JMRI 2007; 26: 823-837.
- Durmo F, et al: Brain tumor characterization using multibiometric evaluation of MRI. Tomoy 2018; 4: 14-25.
- Port M, et al: Efficiency, thermodynamic and kinetic stability of marketed gadolinium chelates and their possible clinical consequences: a critical review. Biometals 2008; 21: 469-490.
- Matoori S, Gutzeit A, Fröhlich JM: Nephrogenic systemic fibrosis in 2015. Switzerland Med Forum 2015; 15: 340-344.
- Grobner T: Gadolinium – a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant 2006; 21: 1104-1108.
- Cowper SE, et al: Scleromyxoedema-like cutaneous diseases in renal-dialysis patients. Lancet 2000; 356: 1000-1001.
- Cowper SE: Nephrogenic fibrosing dermopathy: the first 6 years. Curr Opin Rheumatol 2003; 15: 785-790.
- Girardi M, et al: Nephrogenic systemic fibrosis: clinicopathological definition and workup recommendations. J Am Acad Dermatol 2011; 65: 1095-1106.
- Grebe S, et al: Chronic inflammation and accelerated atherosclerosis as important cofactors in nephrogenic systemic fibrosis following intravenous gadolinium exposure. Clin Exp Nephrol 2008; 12: 403-406.
- Bennett Cl, et al: Gadolinium-induced nephrogenic systemic fibrosis: the rise and fall of an iatrogenic disease. Clin Kidney J 2012; 5: 82-88.
- Kanda T, et al: High Signal Intensity in the Dentate Nucleus and Globus Pallidus on Unenhanced T1-weighted MR Images: Relationship with Increasing Cumulative Dose of a Gadolinium-based Contrast Material. Radiology 2014; 270(3): 834-841.
- Pozeg P, et al: Spatio-temporal pattern of gadolinium-related hyperintensity increase within deep brain nuclei. Swiss Congress of Radiology, Lausanne May 10-12, 2018; SS149.
- McDonald RJ, et al: Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 2015; 275: 772-782.
- European Medicines Agency EMA. EMA’s final opinion confirms restrictions on use of linear gadolinium agents in body scans. www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/referrals/Gadolinium-containing_contrast_agents/human_referral_prac_000056.jsp (accessed May 22, 2018).
- Murata N, Murata K, Gonzalez-Cuyar LF, Maravilla MR: Gadolinium tissue deposition in brain and bone. Magn Reson Imaging 2016; 34: 1359-1365.
- McDonald RJ, et al: Comparison of gadolinium concentrations within multiple rat organs after intravenous administration of linear versus macrocyclic gadolinium chelates. Radiology 2017; 285: 536-545.
- Ramalho M, Ramalho J, Burke LM, Semelka RC: Gadolinium retention and toxicity – an update. Adv Chronic Kidney Dis 2017; 24: 138-146.
- Berger F, et al: Gadolinium-distribution in cerebrospinal fluid after administration of a gadolinium-based MR contrast agent in humans. Radiology 2018; https://doi.org/10.1148/radiol.2018171829.
- Rogosnitzky M, Branch S: Gd-based contrast agent toxicity: a review of known and proposed mechanisms. Biometals 2016; 29: 365-376.
InFo NEUROLOGY & PSYCHIATRY 2018; 16(4): 23-29.