Numerous studies have been published regarding new options for complementary medicine treatment for side effects of antihormonal therapies. Some of the affected patients may benefit from complementary medicine treatment options. These measures may help to maximize the potential of anti-hormonal oncologic treatments.
In 2002, the World Health Organization (WHO) called for greater recognition of complementary medicine and natural therapies and developed a program to this end. The director of the WHO at that time, Jonathan Quick, presented the following plan of the WHO: A collection of evidence on the efficacy, quality and safety of naturopathy and complementary medicine should be established. The aim was to complement conventional medicine with complementary medicine. Proven alternatives to conventional treatments should be recognized and promoted by each country’s public health system.
This WHO program and other developments have contributed to more intensive research into natural remedies and complementary medicine, both in basic research and in preclinical and clinical research. As a result of this research, the number of scientific publications in this field has increased significantly. Complementary to the quantity, the quality of the studies performed has also increased, which were increasingly conducted according to the criteria of evidence-based medicine, recognizable, among other things, by the growing placement of publications in peer-reviewed journals with high impact factors. Thus, a growing body of serious knowledge has emerged in the field of complementary medicine and naturopathic medicine.
Traditional and complementary medicine
For a long time, so-called evidence-based medicine (EBM) was contrasted with complementary and alternative medicine (CAM) as the antithesis. Then it was recognized that complementary medicine can also be evidence-based, thus eliminating the basis for polarization between EBM and CAM. Furthermore, it was recognized that complementary medicine and alternative medicine are two different things and should not be lumped together in one collective term (CAM).
As a consequence, the WHO abandoned the collective term CAM in 2014 and introduced the following nomenclature instead: T&CM = Traditional and Complementary Medicine. To further develop this topic, WHO has published a corresponding program, entitled: WHO Traditional Medicine Strategy 2014-2023 [1].
Change in oncological therapy concepts
In parallel with the WHO initiative, the content of oncological therapy concepts in conventional medicine has changed significantly. This is particularly true for anti-hormonal therapy in patients with hormone-dependent tumors, and especially for patients with breast carcinoma with evidence of estrogen and/or progesterone receptors in tumor tissue.
The increasing establishment of endocrine therapy with aromatase inhibitors and the growing use of GnRH analogues reflect changes in treatment concepts. The strategy of extended adjuvant antihormonal therapy (EAT) for an additional two years after five years of antihormonal therapy, as well as the concept of extending treatment with tamoxifen from five to ten years, also demonstrates that antihormonal therapy has gained in importance.
Similarly, the “switch concept” in terms of treatment of tamoxifen and aromatase inhibitors in different sequences is an expression of the further differentiation of anti-hormonal therapies.
Anti-hormonal therapies in breast carcinoma
Breast carcinoma is one of the most common carcinomas in women worldwide and one of the largest contributors to oncology-related causes of death [2].
Up to 75% of patients with breast cancer have a tumor that is positive for gene receptor and/or progesterone receptors. This is the basis for anti-hormonal therapy for these women. Depending on age, menopausal status and individual oncological findings of the patient, different endocrine active substances are used in case of receptor positivity.
The best known is tamoxifen, with which the longest experience to date exists, in both pre- and postmenopausal patients. For more than ten years, the aromatase inhibitors anastrozole, exemestane and letrozole have been in use, approved for postmenopausal patients with breast cancer. Furthermore, GnRH analogues for ovarian ablation are used for anti-hormonal therapy, depending on the clinical situation. Most patients with breast carcinoma are postmenopausal at the time of diagnosis or are entering menopause due to systemic oncologic therapy.
Aromatase inhibitors have been studied in prospective randomized clinical trials and compared with tamoxifen. This showed benefits in terms of oncological outcomes of treatment, particularly in high-risk patients, when an aromatase inhibitor was used instead of tamoxifen [3–6]. Also based on these studies, aromatase inhibitors have become a standard of anti-hormonal therapy in patients with postmenopausal breast cancer and have almost completely replaced tamoxifen in this situation. With the increasing use of aromatase inhibitors instead of tamoxifen, there has been a shift in the adverse effects (ADEs) of anti-hormonal therapy to the usually more intense ADEs of aromatase inhibitors from a physician’s perspective. This has become increasingly evident during office hours in recent years.
Side effects of aromatase inhibitors may include musculoskeletal and urogenital symptoms. As a result, the patient’s quality of life may be severely limited, which may lead to discontinuation of therapy. The main focus is usually arthralgia.
Aromatase inhibitors and joint complaints
Aromatase inhibitors cause aromatase inhibitor-associated arthralgia syndrome (AIA) in a proportion of treated patients. The criteria of this syndrome are summarized in Table 1.
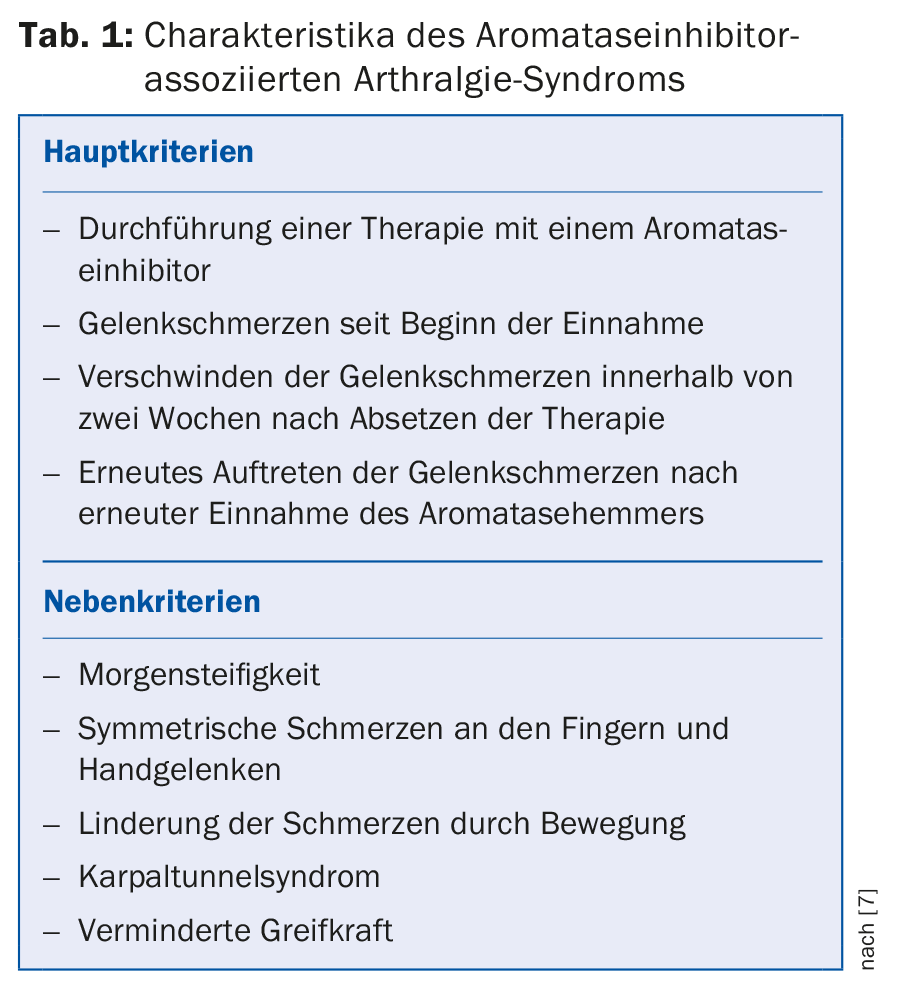
Morales et al. [7] described in a study the short-term intra-articular and tendosynovial changes in aromatase inhibitor-induced arthralgia syndrome. They found inflammatory tendo- and arthrosynovitis with synovial thickening and joint effusions in MRI diagnostics as a correlate to the complaints.
Lombard et al. [8] evaluated AIA as a significant problem with limited treatment options. They found musculoskeletal symptoms in 302 of 370 patients surveyed who were taking an aromatase inhibitor, corresponding to a rate of 82%. 27% of women with musculoskeletal symptoms had discontinued therapy, including 68% because of discomfort. 81% of those affected tried at least one of the following treatment options: 1) Medication prescribed by a doctor, 2) over-the-counter preparations or 3) non-drug measures. The most successful options in the three categories mentioned were: ad 1) anti-inflammatory substances, ad 2) Paracetamol and ad 3) Yoga. One-third of symptomatic patients reported that the use of at least one of these measures prevented discontinuation of the aromatase inhibitor.
Since therapy with aromatase inhibitors is planned for several years, concomitant continuous medication with analgesics and or anti-inflammatory drugs is not a solution for most patients with AIA. The following sections highlight which complementary medicine treatment options exist in principle and which of these options have studies with evidence to demonstrate efficacy for patients with AIA. The goal of these complementary medical therapy options is, on the one hand, that the patient can carry out or continue the aromatase inhibitor therapy as planned (compliance) and, on the other hand, that the best possible quality of life is achieved.
Vitamin D deficiency
Hypovitaminosis D is a predictor of the development of musculoskeletal symptoms in patients taking aromatase inhibitors. This is the key message of a study published in 2014 [9]. Of the 52 patients with aromatase inhibitor use and musculoskeletal complaints, 28 (54%) had hypovitaminosis D. Rheumatologic examination revealed tendinitis in 13 women. Hypovitaminosis D was seen in 33% of patients with levels <40 ng/ml. 19.3% had levels <30 ng/ml and 5.8% had vitamin D levels <20 ng/ml. Symptomatic patients were more likely to have low vitamin D levels compared to asymptomatic patients (p=0.037). In a multivariate regression analysis, patients with vitamin D levels <40 ng/ml had a significantly increased risk of developing tendosynovitis but not myalgia (p=0.033).
Servitja et al. [10] indicated that arthralgias and osteoporosis occur more frequently during therapy with an aromatase inhibitor than during treatment with tamoxifen. The authors found vitamin D levels of <30 ng/ml in 88% of the patients studied who were taking an aromatase inhibitor. In addition, the authors found a close correlation between vitamin D levels and the intensity of arthralgias. As a conclusion, both studies recommend vitamin D substitution when levels are too low.
Arul et al. [11] published in 2016 the results of a study on the efficacy of vitamin D supplementation on the side effect profile of patients with breast cancer treated with the aromatase inhibitor letrozole. Patients with hypovitaminosis D had more severe musculoskeletal symptoms compared with women with normal vitamin D levels. A 12-week supplementation with vitamin D resulted in an increase in vitamin D levels and a decrease in joint symptoms.
Therefore, if aromatase inhibitor-associated arthralgia is suspected, vitamin D levels should always be looked at (Table 2).

Movement
Numerous studies show that exercise can lead to relief of musculoskeletal symptoms in patients with AIA. The HOPE study published in 2016 [12] demonstrates the efficacy of exercise on reducing aromatase inhibitor-associated arthralgia. This was a prospective study conducted over the course of one year. In the exercise study arm, 61 women underwent the following training: moderate to intense aerobic exercise lasting 150 minutes per week, supplemented by strength training, which occurred twice per week. With this study, the positive results of Irwin et al. [13] from 2015, which demonstrated that there is significant improvement in AI-induced arthralgia in previously inactive breast cancer patients with regular exercise.
Another study reviewed the role of Nordic walking in patients with AIA [14]. The authors found that this approach was firstly feasible in patients with AIA and secondly resulted in relief of symptoms.
From the aforementioned studies, it appears that all patients with AIA should be evaluated to determine if a structured exercise program is an option.
Yoga
The overwhelming majority of study results show that yoga is feasible in patients with AIA and can relieve joint pain [15,16].
Acupuncture
The value of acupuncture in patients with AIA has been investigated in several studies. Chen et al. [17] published a meta-analysis on the efficacy of acupuncture for AIA in 2017. Only prospective studies were evaluated. On the basis of five studies with a total of 181 patients, an efficacy of acupuncture was found for complaints existing as a result of AIA. A significant reduction in pain was found after 6-8 weeks of therapy.
Proteolytic enzymes
There is extensive study data on the use of proteolytic enzymes in arthritic conditions, showing positive experience in this area [18,19]. Bromelain is a complex mixture of different proteases. It is obtained from the pressed juice of the pineapple and the stalk of the plant with subsequent ultracentrifugation and freeze-drying. It is available as a drug and approved as an adjuvant for soft tissue inflammation with marked edema formation due to its antiphlogistic properties.
In a prospective double-blind study in patients with osteoarthritis of the knee, bromelain was shown to be equally effective as diclofenac [20].
The scientific working group NATUM (Working Group for Naturopathy, Acupuncture, Environmental and Complementary Medicine in the German Society for Gynecology and Obstetrics) conducted a pilot study with bromelain in patients with AIA [21]. This was a prospective observational study in 21 patients with breast carcinoma and taking an aromatase inhibitor with the following results: Bromelain intake (dose: 8000 F.I.P. per day) reduced the pain intensity of an AIA. There were significant improvements in general activity, walking ability, and quality of life. With the additional intake of bromelain, no patient had to discontinue therapy with the aromatase inhibitor.
Omega-3 fatty acids
The intake of omega-3 fatty acids can relieve arthralgia in patients with arthritis. The American South West Oncology Group (SWOG) investigated the efficacy of omega-3 fatty acid therapy on symptoms of AIA in a placebo-controlled multicenter study (study SWOG S0927). The authors found significant and durable improvement in symptoms of AIA in 249 patients in both the verum and placebo groups. However, no significant difference was found between patients in the verum group and those in the placebo group [22].
Take-Home Messages
- Side effects of anti-hormonal treatments for breast cancer can reduce quality of life, leading to discontinuation of therapy.
- In recent years, numerous studies have been published regarding new options for complementary medicine treatment for side effects of anti-hormonal treatments.
- A proportion of patients suffering from side effects of anti-hormonal therapies may benefit from complementary medicine treatment options.
- The quality of life of affected women can be improved and premature discontinuation of anti-hormonal treatment can be avoided.
- Complementary medicine interventions can help to maximize the potential of anti-hormonal oncologic treatments.
Literature:
- WHO Traditional Medicine Strategy 2014-2023, Geneva, WHO 2014.
- Jemal A, et al: Cancer statistics, 2009. CA Cancer J Clin 2009; 59: 225-249.
- Burstein HJ, et al: American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol 2010; 28: 3784-3796.
- Boccardo F, et al: Switching to anastrozole versus continued tamoxifen treatment of early breast cancer: long term results of the Italian Tamoxifen Anastrozole trial. Eur J Cancer 2013; 49: 1546-1554.
- Boccardo F, et al: Switching to anastrozole versus continued tamoxifen treatment of early breast cancer. Updated results of the Italian tamoxifen anastrozole (ITA) trial. Ann Oncol 2006; 17: vii10-14.
- Boccardo F, et al: Switching to anastrozole versus continued tamoxifen treatment of early breast cancer: preliminary results of the Italian Tamoxifen Anastrozole Trial. J Clin Oncol 2005; 23: 5138-5147.
- Morales L, et al: Prospective study to assess short-term intra-articular and tenosynovial changes in the aromatase inhibitor-associated arthralgia syndrome. J Clin Oncol 2008; 26(19): 3147-1352.
- Lombard JM et al: Aromatase inhibitor-induced musculoskeletal syndrome: a significant problem with limited treatment options. Support Care Cancer 2016; 24(5): 2139-2146.
- Singer O et al. Hypovitaminosis D is a predictor of aromatase inhibitor musculoskeletal symptoms. Breast J 2014; 20(2): 174-179.
- Servitja S, et al: Skeletal adverse effects with aromatase inhibitors in early breast cancer: evidence to date and clinical guidance. Ther Adv Med Oncol 2015; 7(5): 291-296.
- Arul Vijaya Vani S, et al: Effects of vitamin D and calcium supplementation on side effects profile in patients of breast cancer treated with letrozole.Clin Chim Acta 2016; 459: 53-56.
- Arem H, et al: Exercise adherence in a randomized trial of exercise on aromatase inhibitor arthralgias in breast cancer survivors: the Hormones and Physical Exercise (HOPE) study. J Cancer Surviv 2016; 10(4): 654-662.
- Irwin ML, et al: Randomized exercise trial of aromatase inhibitor-induced arthralgia in breast cancer survivors. J Clin Oncol 2015; 33(10): 1104-1111.
- Fields J, et al: Nordic Walking as an Exercise Intervention to Reduce Pain in Women With Aromatase Inhibitor-Associated Arthralgia: A Feasibility Study. J Pain Symptom Manage 2016 Oct; 52(4): 548-559.
- Jacobsen PB, et al: Pilot study of Iyengar yoga for management of aromatase inhibitor-associated arthralgia in women with breast cancer. Psychooncology 2015; 24(11): 1578-1580.
- Peppone LJ, et al: The effect of YOCAS©® yoga for musculoskeletal symptoms among breast cancer survivors on hormonal therapy. Breast Cancer Res Treat 2015; 150(3): 597-604.
- Chen L, et al: Effect of acupuncture on aromatase inhibitor-induced arthralgia in patients with breast cancer: A meta-analysis of randomized controlled trials. Breast 2017; 33: 132-138.
- Brien S, et al: Bromelain as an adjunctive treatment for moderate-to-severe osteoarthritis of the knee: a randomized placebo-controlled pilot study. QJM 2006; 99(12): 841-850.
- Walker AF, et al: Bromelain reduces mild acute knee pain and improves well-being in a dose-dependent fashion in an open study of otherwise healthy adults. Phytomedicine 2002; 9(8): 681-686.
- Akhtar NM, et al. Oral enzyme combination versus diclofenac in the treatment of osteoarthritis of the knee-a double-blind prospective randomized study. Clin Rheumatol 2004; 23(5): 410-415. Epub 2004 Jul 24.
- Wagner S: With bromelain against arthalgia complaints. Gynecology + Obstetrics 2015; 20(2): 50-51.
- Hershman DL, et al: Randomized Multicenter Placebo-Controlled Trial of Omega-3 Fatty Acids for the Control of Aromatase Inhibitor-Induced Musculoskeletal Pain: SWOG S0927. J Clin Oncol 2015; 33(17): 1910-1917.
HAUSARZT PRAXIS 2017; 12(9): 30-34