Only 5-10% of patients with hypertension have secondary hypertension. In young patients (<30 years) we recommend screening, as cure of hypertension can be achieved by treating the cause. Due to the expensive and time-consuming nature of screening, we recommend that this be done only in selected cases. Pseudoresistance should be excluded before screening with 24-h ambulatory blood pressure measurement. The most common causes of secondary hypertension are obstructive sleep apnea, renal parenchymal disease, renovascular disease, and primary hyperaldosteronism.
Secondary hypertension is defined as elevated systemic blood pressure (BP) due to an identifiable cause. The prevalence of secondary hypertension varies depending on the patient population studied and the intensity of diagnosis. However, 90-95% of all patients with arterial hypertension have an essential form (idiopathic or primary) [1]. Therefore, in order to improve cost-effectiveness, not every patient with elevated BP should be screened for secondary forms by means of an elaborate and expensive screening procedure. Young patients, those with refractory hypertension, or those without a family history of arterial hypertension are much more likely to have secondary hypertension. Early identification and specific therapy of secondary hypertension can at best, but rarely, lead to normalization of BP or, most often, to a better response to drug therapy, thus freeing the patient from many years of polymedication.
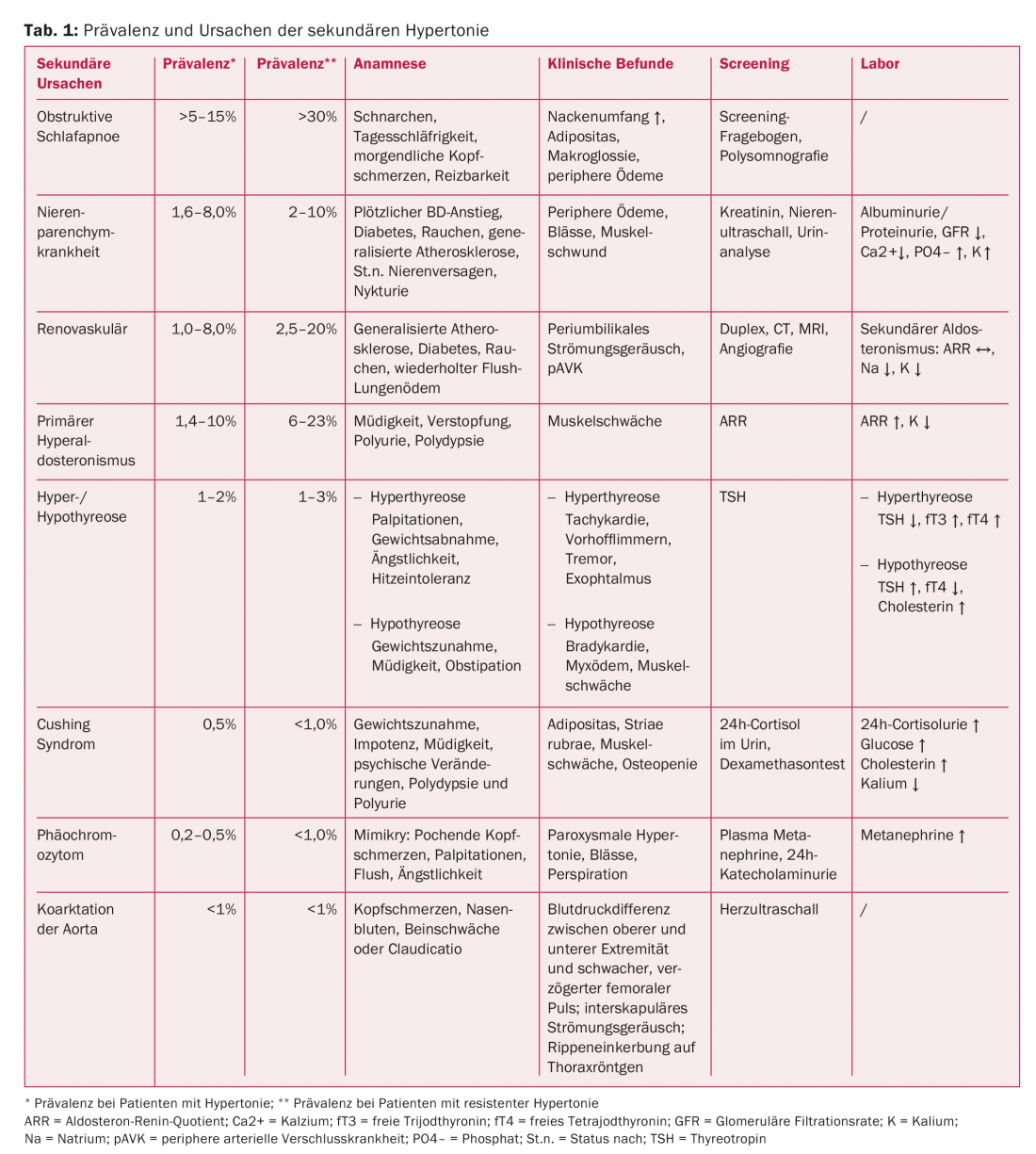
Arterial hypertension is the most common and important cardiovascular risk factor in adults with increasing prevalence with age. More than 50% of the population between 55 and 64 years suffer from arterial hypertension [2,3]. An identifiable cause is found in only 5-10% of patients. The prevalence of secondary hypertension varies depending on the patient population studied and the screening method used to identify a cause [4–7]. In patients with refractory (resistant) hypertension, the prevalence of a secondary cause is significantly higher compared with patients with well-controlled BP (Table 1) [8]. Resistant hypertension is when BP is elevated despite the use of three antihypertensives including a thiazide diuretic under optimal dosing.
Who should be examined?
General clinical clues indicating the presence of a secondary form of hypertension are listed in Table 2.
Age: Hypertension in prepubertal children is usually due to renal disease (renoparenchymatous or renovascular) or coarctation of the aorta [9]. Young adults (<30 years) without family history or other risk factors should be screened.
Habitus: obese patients with resistant hypertension should be evaluated for obstructive sleep apnea (OSA), the most common secondary cause in adults [10], and hypercortisolism/hypothyroidism.
BP: Patients in need of clarification are those with resistant hypertension, severe hypertension at initial presentation (>180/110 mmHg), hypertensive emergencies [5], absence of a nocturnal BP drop (“dipping”) of >10% relative to the daytime value during a 24-hour ambulatory BP measurement (ABPM), or increase in nocturnal BP (“reverse dipper”) [11,12].
Generalized atherosclerosis: only about 15-30% of patients with arterial hypertension and diffuse atherosclerosis have significant renal artery stenosis (defined as ≥50%) [13,14]. Other indications include resistant hypertension, a sudden BP rise in previously well-controlled hypertension, severe hypertension, or nondipping.

What should be excluded before screening?
Pseudo-resistance should be ruled out before starting screening [8]. Common causes include:
Inadequate blood pressure measurement: the patient did not sit still for five minutes beforehand or an incorrect cuff size was selected; a size that is too small can lead to incorrectly high BP values [15].
Insufficient treatment control: On the one hand, physicians control therapy too little (“therapeutic inertia”) because they are not aware that hypertension must be treated consistently [16]. As a result, therapy is not adjusted to the target BP values according to guidelines.
Lack of treatment adherence: on the other hand, patients show insufficient adherence, with only 40% continuing with their prescribed treatment after ten years of follow-up [17]. In 50% of patients with resistant hypertension, urinalysis can demonstrate that they did not take their medication as prescribed [18]. In many patients with resistant hypertension, BP normalizes as soon as adherence is controlled or medication is administered via electronic dosage [19]. Various strategies for improvement, such as measurement of drug concentration in blood/urine, electronic dosage, or support programs were discussed. A simple option is also to take the medication under supervision and then perform 24-hour ambulatory blood pressure monitoring [20].
White coat hypertension: this is a practice BP >140/90 mmHg, whereas BP values in ABPM are in the normal range. This is a common cause of pseudo-resistance and is present in up to 30% of patients with elevated practice BP [21]. Therefore, 24-h ABPM must be performed in any patient with suspected secondary hypertension.
Drug-induced hypertension: drugs such as NSAIDs, glucocorticoids, appetite suppressants, stimulants, mucosal decongestants, or licorice can lead to treatment resistance [22,23].
In addition, there is pseudohypertension, which is defined as a 15 mmHg difference in diastolic BP between cuff measurement and contemporaneous intra-arterial measurement [61]. This may be found due to calcified and rigid vessels in elderly patients with arterial hypertension without end organ damage [16,20].
Clarifications for secondary hypertension
Basic diagnostics for hypertension include history, physical examination, blood analysis, and urinalysis. This already provides a first suspicion of the most common secondary forms of hypertension. In Figure 1, we propose a clarification algorithm for suspected secondary causes of hypertension.
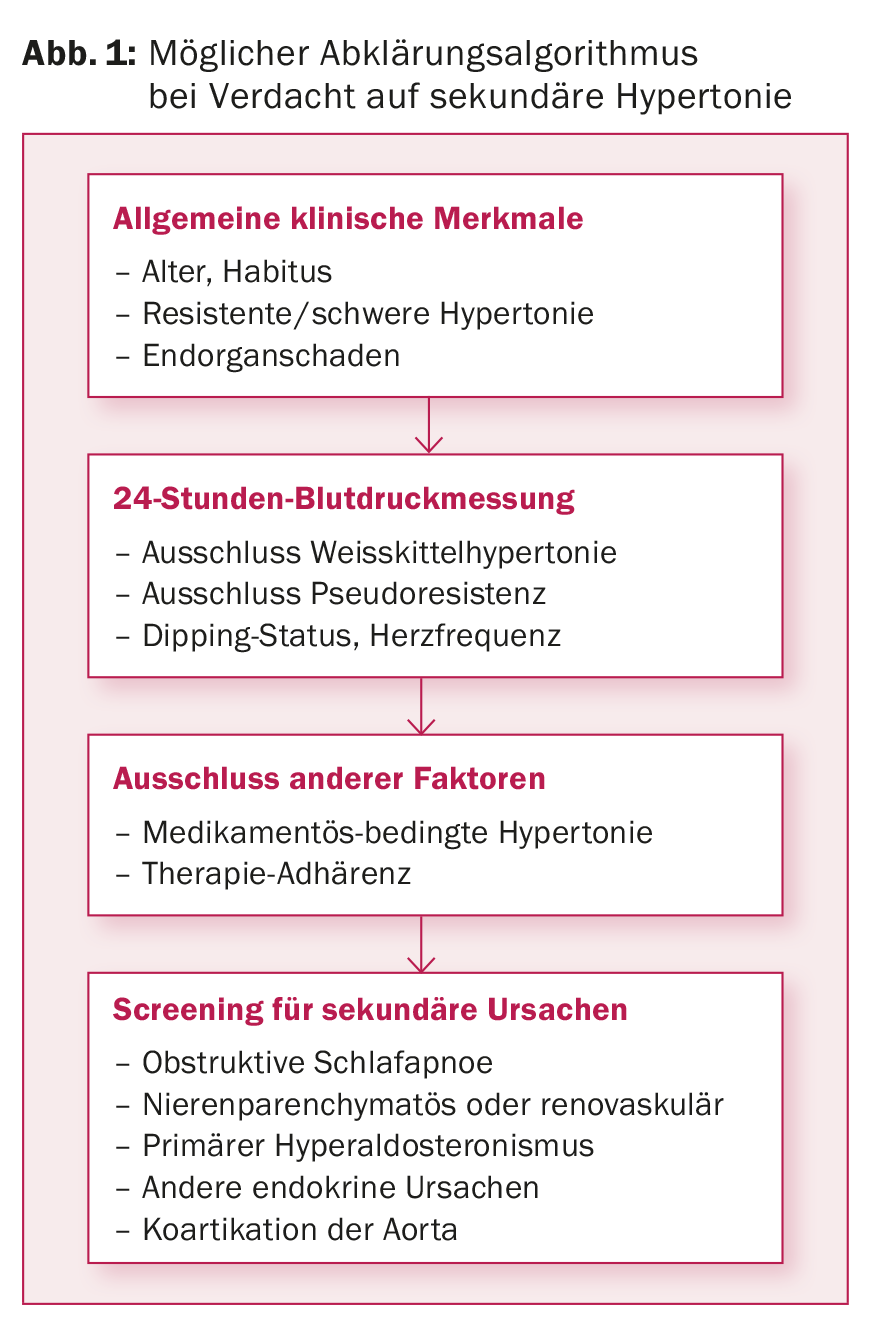
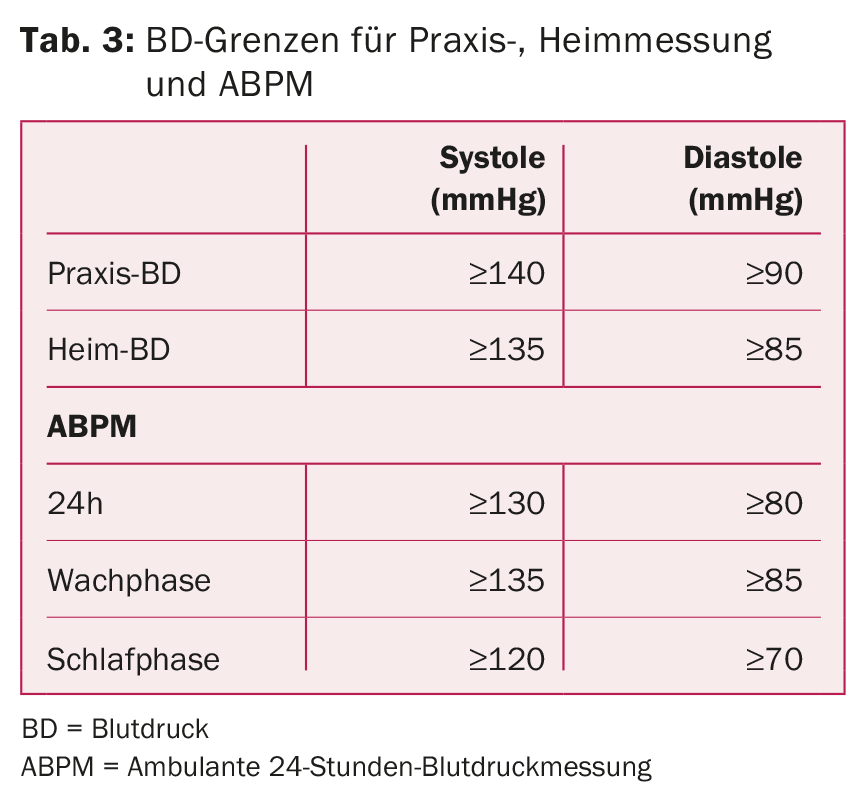
24-Hour Ambulatory BP Measurement: ABPM is the gold standard for assessing arterial BP [12]. White coat hypertension can thus be excluded, adherence to therapy can be monitored, the presence of resistant hypertension can be detected, and nocturnal dipping status can be assessed. Nocturnal reverse dipping with possible increased heart rate suggest the presence of a secondary form (e.g., OSA, renal artery stenosis). Table 3 lists the target blood pressure values.
Obstructive sleep apnea: This is the most common cause of secondary hypertension [10,24] and is classified as mild (AHI 5-15/h), moderate (AHI 16-30/h), and severe (AHI>30/h) based on the apnea-hypopnea index (AHI, number of apneas plus hypopneas per hour of sleep). The cause is a collapse of the upper airway during sleep, which causes recurrent obstructive apneas and hypopneas. Factors leading to BP elevation include increased sympathetic activity, activation of the renin-aldosterone system due to repetitive hypoxemia, and endothelial dysfunction caused by oxidative stress [25–32].
Typical symptoms and clinic are described in Table 1 [33]. With appropriate clinical presentation, an Epworth Sleepiness Scale should be performed [34,35] and with a score ≥10 and a high clinical suspicion of OSA should be clarified by outpatient polygraphy or polysomnography [36]. Continuous positive airway pressure (CPAP) therapy has been shown to decrease BP in several studies [37–40].
Renal parenchymal diseases: Renal parenchymal disease (glomerular, e.g., glomerulonephritis, interstitial, e.g., polycystic kidney disease, or microvascular) is the most common cause of secondary hypertension in children and the second most common in adults [9,41]. Screening is performed by testing for albuminuria/proteinuria, as well as serum creatinine. If both are normal, the likelihood of the presence of significant renal parenchymal disease is very low. If pathologic, sonography of the kidneys is indicated to evaluate size, shape, renal mass, and to exclude urinary tract obstruction.
Renovascular disease: in children and young adults, fibromuscular dysplasia is the most common cause of secondary hypertension and should be excluded by imaging studies (screening with duplex ultrasound, confirmation by angiography). If fibromuscular dysplasia is confirmed, MR angiography of the cerebrovascular vessels is indicated [42]. In adults, on the other hand, arteriosclerotic renal artery stenosis (NAS) is the most common form [13], especially common in hypertensive patients with generalized atherosclerosis [43–45]. Clinical clues include worsening renal function on angiotensin-converting enzyme inhibitors (ACE inhibitors) or angiotensin receptor blockers (ARBs), severe hypertension or sudden elevation of blood pressure especially in smokers, diabetics with diffuse atherosclerosis, recurrent flash pulmonary edema [46,47], and periumbilical flow murmur. In patients with evidence of the presence of RAS and likely favorable response to revascularization (Table 4), we recommend imaging (duplex ultrasound, CT, or MRI) [48] and, if confirmed, performing hemodynamic measurement to demonstrate a significant gradient. Clinically, RAS associated with arterial hypertension should be distinguished from RAS causative of arterial hypertension [49]. The longer a RAS has been present, the less likely normalization of BP can be achieved after correction [49].

Primary hyperaldosteronism: this is defined as increased aldosterone production independent of the renin-angiotensin system, which cannot be suppressed by sodium loading. The cause is usually an adrenal adenoma, unilateral or bilateral adrenal hyperplasia, or glucocorticoid-dependent hyperaldosteronism. Hyperaldosteronism is suspected in patients with repeated hypokalemia (but present in only about 40% of patients) [50], excessive hypokalemia despite small doses of diuretics, resistant hypertension, muscle weakness, constipation and fatigue, metabolic alkalosis, excessive sodium excretion, or concomitant hypernatremia.
The aldosterone-renin quotient can be used as a screening method. However, various factors such as antihypertensives can influence this [51]. The diagnosis should be confirmed by sodium loading test or captopril suppression test [52]. First can be done with measurement of plasma aldosterone before and after infusion of 2000 ml NaCl 0.9% for four hours. An aldosterone concentration <5 ng/dl after infusion argues against, an aldosterone concentration >10 ng/dl for hyperaldosteronism. A decrease in plasma aldosterone >30% of baseline three hours after taking 25-50 mg captopril is suggestive of secondary hyperaldosteronism (essential or renovascular hypertension). For subtype differentiation, it is recommended to perform side-separated adrenal vein catheterization after confirmation by diagnostic imaging (CT/MRI). A unilateral adenoma can be operated minimally invasively, and bilateral disease can be treated with mineral corticoid antagonists.
Rare causes of secondary hypertension
Cushing’s syndrome: In patients with typical habitus (obesity, facial fullness, bull neck, hirsutism and striae rubrae) [53,54], Cushing’s syndrome must be considered. Appropriate screening e.g. by 1 mg dexamethasone test [55] or 24-hour cortisol excretion must be performed. If screening is positive, referral to a specialized center is recommended.
Hyper-/hypothyroidism: Hypothyroidism is indicated by the corresponding clinical signs and elevated diastolic blood pressure (low output and compensation to maintain perfusion by peripheral vasoconstriction), whereas hyperthyroidism is more likely to be associated with elevated systolic blood pressure values.
Pheochromocytoma: This is a very rare cause of secondary hypertension. Clinical features are the five P’s: paroxysmal hypertension, palpitations, perspiration, pallor, and throbbing headache [56]. Screening is recommended in resistant hypertension, appropriate clinic, matching family history, or adrenal tumors and can be performed by 24-h urine of metanephrine/normetanephrine or determination of free metanephrines in plasma [57]. If positive, we recommend referral to a specialized center.
Coarctation of the aorta/aortic isthmus stenosis: this is the second most common cause of hypertension in children and young adults [9,58]. Typical symptoms are headaches, cold feet and pain in the legs during physical activity. Clinical findings include weak femoral pulses, a systolic blood pressure differential between the right arm and right leg, and a systolic. For screening, we recommend echocardiography. In this population, rigorous long-term follow-up is indicated even after treatment [59,60].
Literature:
- Mancia G, et al: 2013 ESH/ESC Guidelines for the management of arterial hypertension. The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). European Heart Journal 2013: 34; 2159-2219.
- Roger VL, et al: Heart disease and stroke statistics-2011 update: A report from the American heart association. Circulation 2011; 123: e18-e209.
- Wolf-Maier K, et al: Hypertension prevalence and blood pressure levels in 6 European countries, Canada, and the United States. JAMA 2003; 289: 2363-2369.
- Anderson GH, et al: The effect of age on prevalence of secondary forms of hypertension in 4429 consecutively referred patients. Journal of Hypertension 1994; 12: 609-615.
- Borgel J, et al: Unrecognized secondary causes of hypertension in patients with hypertensive urgency/emergency: prevalence and co-prevalence. Clinical Research in Cardiology 2010; 99: 499-506.
- Omura M, et al: Prospective study on the prevalence of secondary hypertension among hypertensive patients visiting a general outpatient clinic in japan. Hypertension Research 2004; 27: 193-202.
- Sinclair AM, et al: Secondary hypertension in a blood pressure clinic. Archives of Internal Medicine. 1987; 147: 1289-1293.
- Calhoun DA, et al: Resistant hypertension: diagnosis, evaluation, and treatment: A scientific statement from the American heart association professional education committee of the council for high blood pressure research. Circulation 2008; 117: e510-526.
- Arar MY, et al: Etiology of sustained hypertension in children in the southwestern United States. Pediatr Nephrol 1994; 8: 186-189.
- Pedrosa RP, et al: Obstructive sleep apnea: The most common secondary cause of hypertension associated with resistant hypertension. Hypertension 2011; 58: 811-817.
- Davies CW, et al: Case-control study of 24 hour ambulatory blood pressure in patients with obstructive sleep apnea and normal matched control subjects. Thorax 2000; 55: 736-740.
- Pickering TG, et al: Ambulatory blood-pressure monitoring. The New England Journal of Medicine 2006; 354: 2368-2374.
- Garovic VD, Textor SC: Renovascular hypertension and ischemic nephropathy. Circulation 2005; 112: 1362-1374.
- Leertouwer TC, et al: Incidental renal artery stenosis in peripheral vascular disease: A case for treatment? Kidney International 2001; 59: 1480-1483.
- Pickering TG, et al: Recommendations for blood pressure measurement in humans and experimental animals: Part 1: Blood pressure measurement in humans: A statement for professionals from the subcommittee of professional and public education of the American heart association council on high blood pressure research. Circulation 2005; 111: 697-716.
- Rimoldi SF, et al: Secondary arterial hypertension: when, who and how to screen? European Heart Journal 2014; 35: 1245-1254.
- Van Wijk BL, et al: Rate and determinants of 10-year persistence with antihypertensive drugs. Journal of Hypertension 2005; 23: 2101-2107.
- Jung O, et al.: Resistant hypertension? Assessment of adherence by toxicological urine analysis. J Hypertens 2013; 3: 766-774.
- Burnier M, et al: Electronic compliance monitoring in resistant hypertension: the basis for rational therapeutic decisions. J Hypertens 2001; 19: 335-341.
- Rimoldi SF, et al: Resistant hypertension: what the cardiologist needs to know. European Heart Journal 2015; 36: 2686-2695.
- Verdecchia P, et al: White coat hypertension and white coat effect. Similarities and differences. American Journal of Hypertension 1995; 8: 790-798.
- Grossman E, Messerli FH. Drug-induced hypertension: An unappreciated cause of secondary hypertension. Am J Med 2012; 125: 14-22.
- Sigurjonsdottir HA, et al: CSF-induced rise in blood pressure: A linear dose-response relati-onship. Journal of Human Hypertension 2001; 15: 549-552.
- Logan AG, et al: High prevalence of unrecognized sleep apnea in drug-resistant hypertension. Journal of Hypertension 2001; 19: 2271-2277.
- Jurado-Gamez B, et al: Relationship of oxidative stress and endothelial dysfunction in sleep apnea. Eur Respir J 2011; 37: 873-879.
- Fletcher EC: Sympathetic over activity in the etiology of hypertension of obstructive sleep apnea. Sleep 2003; 26: 15-19.
- Leuenberger UA, et al: Effects of intermittent hypoxia on sympathetic activity and blood pressure in humans. Auton Neurosci 2005; 121: 87-93.
- Calhoun DA, et al: Aldosterone excretion among subjects with resistant hypertension and symptoms of sleep apnea. Chest 2004; 125: 112-117.
- Goodfriend TL, Calhoun DA: Resistant hypertension, obesity, sleep apnea, and aldosterone: theory and therapy. Hypertension 2004; 43: 518-524.
- Ip MS, et al: Endothelial function in obstructive sleep apnea and response to treatment. American Journal of Respiratory and Critical Care Medicine 2004; 169: 348-353.
- Rimoldi SF, et al: Systemic vascular dysfunction in patients with chronic mountain sickness. Chest 2012; 141: 139-146.
- Bailey DM, et al: Oxidative-nitrosative stress and systemic vascular function in highlanders with and without exaggerated hypoxemia. Chest 2013; 143: 444-451.
- Alonso-Fernandez A, et al: Cardiac rhythm disturbances and st-segment depression episodes in patients with obstructive sleep apnea-hypopnea syndrome and its mechanisms. Chest 2005; 127: 15-
- Johns MW: A new method for measuring daytime sleepiness: The Epworth-sleepiness scale. Sleep 1991; 14: 540-545.
- Johns MW: Daytime sleepiness, snoring, and obstructive sleep apnea. The Epworth-sleepiness scale. Chest 1993; 103: 30-36.
- Thurnheer R, et al: Respiratory polygraphy in sleep apnea diagnosis. Report of the Swiss respiratory polygraphy registry and systematic review of the literature. Swiss Med Weekly 2007; 137: 97-102.
- Pepperell JC, et al: Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnea: A randomised parallel trial. Lancet 2002; 359: 204-210.
- Becker HF, et al: Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation 2003; 107: 68-73.
- Logan AG, et al. Refractory hypertension and sleep apnea: Effect of cpap on blood pressure and barorefl ex. Eur Respir J 2003; 21: 241-247.
- Suzuki M, et al: Long-term nasal continuous positive airway pressure administration can normalize hypertension in obstructive sleep apnea patients. Sleep 1993; 16: 545-549.
- Whaley-Connell AT, et al: Ckd in the united states: kidney early evaluation program (keep) and national health and nutrition examination survey (nhanes) 1999-2004. American Journal of Kidney Diseases 2008; 51: S13-20.
- Slovut DP, Olin JW: Fibromuscular dysplasia. The New England Journal of Medicine 2004; 350: 1862-1871.
- Kalra PA, et al: Atherosclerotic renovascular disease in united states patients aged 67 years or older: Risk factors, revascularization, and prognosis. Kidney International 2005; 68: 293-301.
- Rimoldi SF, et al: Screening renal artery angiography in hypertensive patients undergoing coronary angiography and 6-month follow-up after ad hoc percutaneous revascularization. Journal of Hypertension 2010; 28: 842-847.
- Textor SC, Lerman L: Renovascular hypertension and ischemic nephropathy. American Journal of Hypertension 2010; 23: 1159-1169.
- Messerli FH, et al: Flash pulmonary oedema and bilateral renal artery stenosis: The pickering syndrome. European Heart Journal 2011; 32: 2231-2235.
- Rimoldi SF, et al: Flash pulmonary edema. Progress in Cardiovascular Diseases 2009; 52: 249-259.
- Baumgartner I, Lerman LO. Renovascular hypertension: screening and modern management. European Heart Journal 2011; 32: 1590-1598.
- Bavishi C, et al. : Atherosclerotic Renal Artery Stenosis and Hypertension: Pragmatism, Pitfalls, and Perspectives. Am J Med 2016; 129(6): 635.e5-635.e14.
- Mulatero P, et al: Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. The Journal of Clinical Endocrinology and Metabolism 2004; 89: 1045-1050.
- Funder JW, et al: Case detection, diagnosis, and treatment of patients with primary aldosteronism: An endocrine society clinical practice guideline. The Journal of Clinical Endocrinology and Metabolism 2008; 93: 3266-3281.
- Mulatero P, et al: Comparison of confirmatory tests for the diagnosis of primary aldosteronism. The Journal of Clinical Endocrinology and Metabolism 2006; 91: 2618-2623.
- Newell-Price J, et al: Cushing’s syndrome. Lancet 2006; 367: 1605-1617.
- Nieman LK, et al: The diagnosis of cushing’s syndrome: An endocrine society clinical practice guideline. The Journal of Clinical Endocrinology and Metabolism 2008; 93: 1526-1540.
- Findling JW, et al: The Journal of Clinical Endocrinology and Metabolism 2004; 89: 1222-1226.
- Young WF, Jr: Adrenal causes of hypertension: pheochromocytoma and primary aldosteronism. Reviews in Endocrine & Metabolic Disorders 2007; 8: 309-320.
- Singh RJ: Advances in metanephrine testing for the diagnosis of pheochromocytoma. Clinics in Laboratory Medicine 2004; 24: 85-103.
- Prisant LM, et al: Coarctation of the aorta: A secondary cause of hypertension. J Clin Hypertens (Greenwich) 2004; 6: 347-350, 352.
- Weber HS, et al: Endovascular stenting for native coarctation of the aorta is an effective alternative to surgical intervention in older children. Congenital Heart Disease 2008; 3: 54-59.
- Stewart AB, et al: Coarctation of the aorta life and health 20-44 years after surgical repair. British Heart Journal 1993; 69: 65-70.
CARDIOVASC 2016; 15(5): 24-28
HAUSARZT PRAXIS 2017; 12(1): 18-23