The carotid stimulator is a device that electrically activates the baroreflex mechanism. As a result, sympathetic tone is attenuated and the renin-angiotensin-aldosterone system is dampened, causing a reduction in blood pressure and heart rate. Baroreflex activation therapy (BAT) leads to a permanent average reduction in systolic blood pressure of 40-50 mmHg after four years in the long-term. In addition to the blood pressure lowering effect, a reduction in heart muscle thickness was observed in the patients. The effect in patients with heart failure is currently being evaluated. Implantation of the device is considered safe. The carotid stimulator is now available in several European countries.
Arterial hypertension, defined as systolic blood pressure above 140 mmHg or diastolic blood pressure above 90 mmHg, affects more than 30% of adults in the Western world. Effective blood pressure treatment results in substantial risk reduction of stroke, myocardial infarction, and heart failure [1,2]. Despite optimized drug therapy and lifestyle changes, only 25-34% of patients achieve a target blood pressure of 140/90 mmHg or below [3]. Many patients have refractory hypertension or suffer from drug-induced side effects.
Drug therapy remains the mainstay of treatment for arterial hypertension. In the last ten years, however, other forms of therapy besides purely medicinal treatment have become public:
- The electrical baroreceptor stimulation
- Biofeedback and other psychosomatic treatments
- The renal artery denervation
- The vaccination against blood pressure (angiotensin antibodies)
- Vagus nerve electrical stimulation.
Both renal artery denervation and blood pressure vaccination failed to gain acceptance [4,5]. And just at the last ESC Congress 2014 in Barcelona, the results of the NECTAR-HF study were presented, which showed that vagus nerve stimulation is not able to improve cardiac function [6].
In contrast, baroreflex activation therapy using a carotid stimulator demonstrated sustained blood pressure reduction in patients with refractory arterial hypertension.
About the history
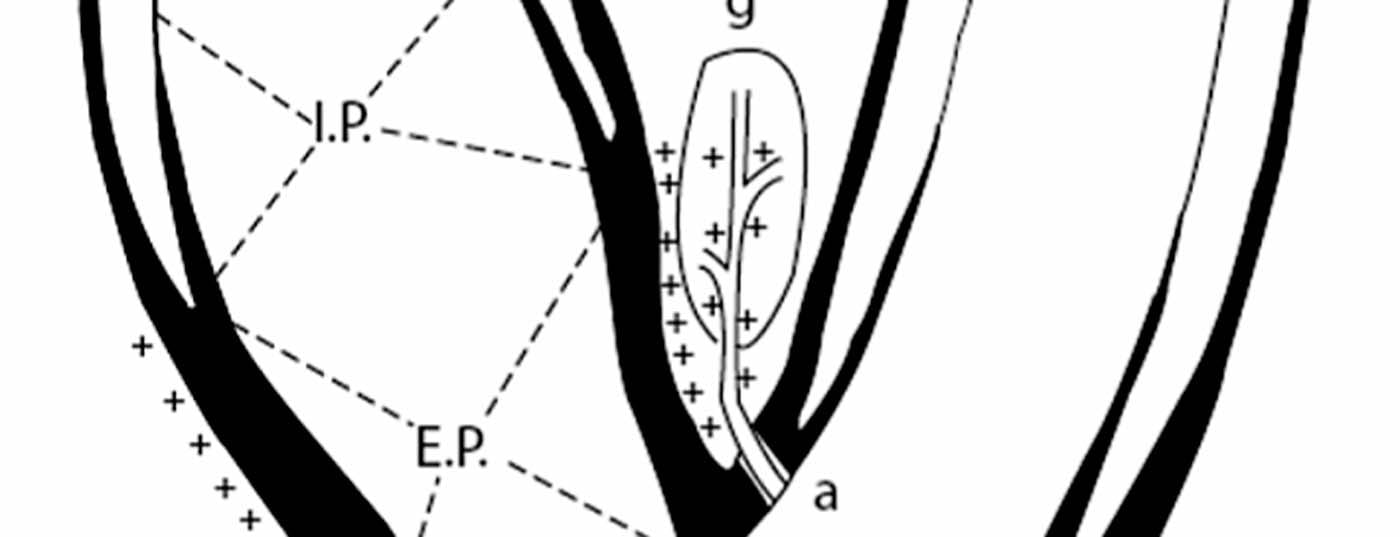
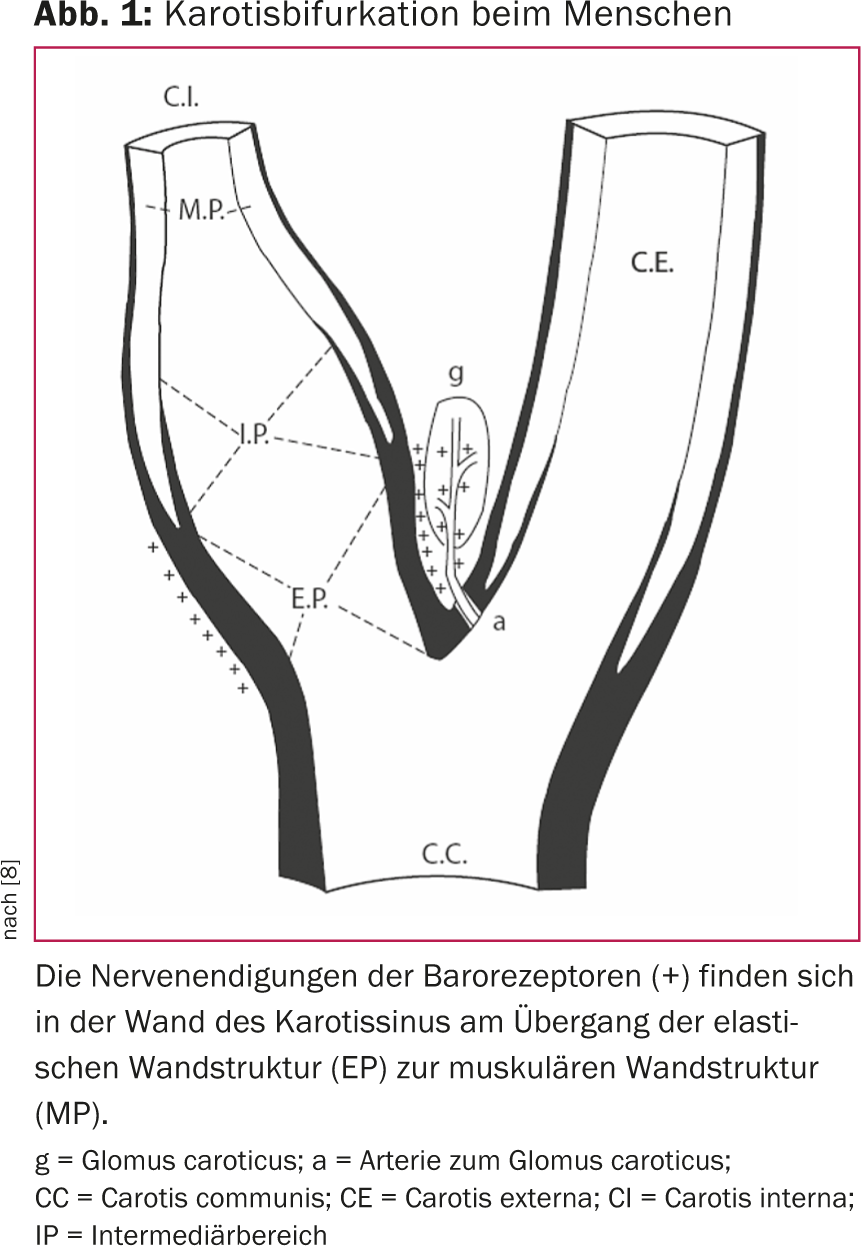
The importance of the baroreflex in blood pressure regulation was recognized more than 80 years ago [7]. In humans, the so-called blood pressure sensors, i.e. the baroreceptors, are mainly located in the aortic arch, in the subclavian arteries and in the region of the carotid sinus. Baroreceptors in the carotid sinus appear to be dominant. The distribution and feedback mechanism of baroreceptor nerve fibers have been intensively studied (Fig. 1) [8]. Essentially, an increase in blood pressure causes increased wall tension in the carotid sinus. This activates the baroreceptors in the carotid sinus wall, resulting in increasing frequency and amplitude of afferent nerve impulses. The impulses are interconnected in the brain and lead, both neuro-humorally and via modulation of the autonomic nervous system, to a dampening of the sympathetic tone. Accordingly, blood pressure is then influenced via cardiac function, via the kidneys and via peripheral vascular resistance (Fig. 2).
As early as the 1960s, carotid stimulators were used in a study in patients with refractory arterial hypertension [9]. However, for technical reasons and because better blood pressure drugs have been developed, this therapeutic approach has been abandoned and the industry has turned its attention to the development of pacemakers. The idea of electrical baroreceptor stimulation was not revived until the beginning of this century. Both animal studies and long-term human studies have shown a positive and lasting effect on blood pressure regulation [10,11].
Device specification and implantation technique
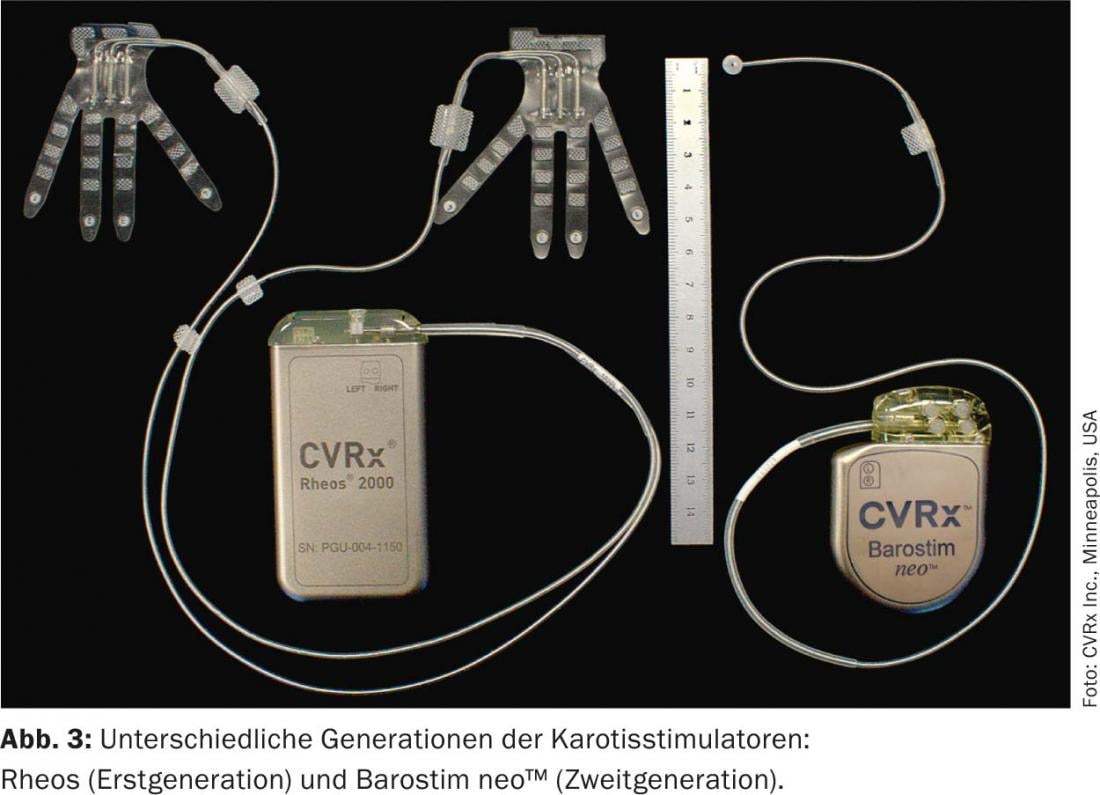
The first generation device (Rheos-System Baroreflex Activation Therapy Device®, CVRx Inc., Minneapolis, MN, USA) required implantation of a generator and two probes. The electrodes were placed around the carotid sinus on both sides. The new generation device (Barostim neo™), on the other hand, has only one probe, which is preferably implanted on the right side. In addition, the generator and electrode have been significantly reduced in size and the battery life has been extended to 48 months on average. In monopolar stimulation, the generator serves as the active electrode (Figs. 3 and 4). In addition, the current is now applied constantly and no longer intermittently.
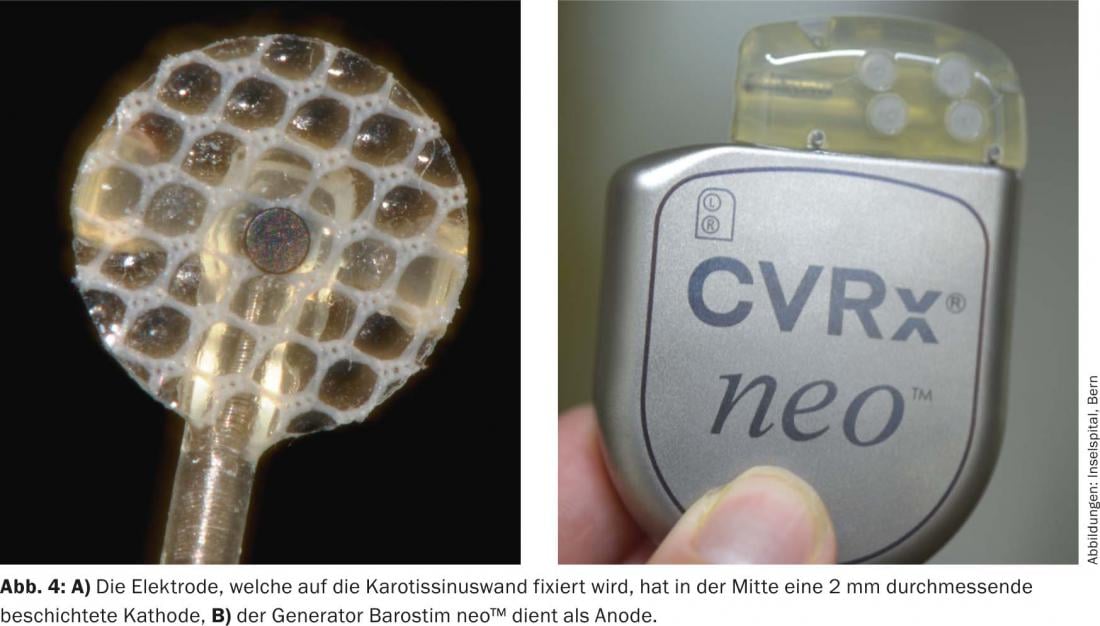
Implantation of the device is performed under anesthesia. The procedure lasts 60-90 minutes. Through a surgical approach over the right carotid bifurcation, the carotid bifurcation and anterior wall of the carotid sinus are visualized. It is then tested in which area of the carotid sinus the strongest response occurs (i.e., lowering of systolic blood pressure and/or pulse). Next, the electrode is fixed with fine sutures. A pocket is formed for the generator infraclavicularly on the right, similar to a pacemaker implantation. The electrode is tunnelled along the carotid artery to the level of the jugulum and then subcutaneously to the generator pocket and connected to the generator. A relief loop is formed in the region of the common carotid artery, which should prevent traction on the electrode when the head is turned. The anesthesia must be planned specifically, since inhalation anesthetics in particular can dampen the baroreflex. The surgery is not stressful and can be done well during a short hospitalization. The device is then not switched on until two to four weeks after implantation. Antihypertensive medication should be continued and, depending on the response of blood pressure to baroreceptor stimulation, can then be gradually reduced during the course.
Clinical studies
Within an acute study (BRASS, BaroReceptor Activation System Study), we electrically activated the baroreflex during common carotid surgery [12]. Significant voltage-dependent blood pressure and pulse reductions were obtained under increasing stimulation. The strongest response was detected when stimulated with 4-6 volts. This reproducibly demonstrated that electrical stimulation of the baroreflex results in an immediate change in blood pressure. This was an important study with regard to permanent implantation of the device to lower blood pressure in patients with refractory hypertension.
Patients with refractory hypertension
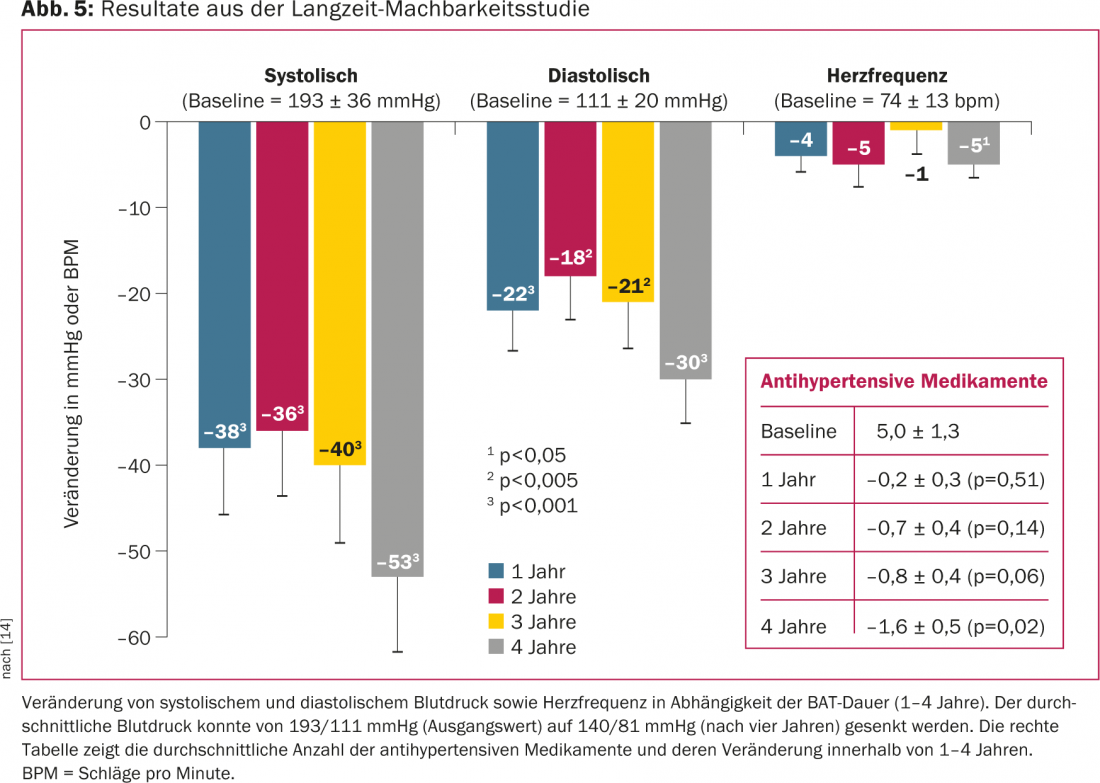
The DEBuT-HT trial(Device Based Therapyin Hypertension Trial) was a prospective, nonrandomized, multicenter European study that evaluated the safety and feasibility of the carotid stimulator in 45 patients with refractory hypertension. Inclusion criterion was a systolic blood pressure above 160 mmHg despite a drug regimen with three antihypertensive drugs, one of which had to be a diuretic [13]. Antihypertensive medication was not changed during the study period. The main end point was a reduction in systolic blood pressure of at least 10 mmHg three months after BAT. Over three years, all serious events and all implantation- and device-related complications were monitored. Before implantation, the mean systolic blood pressure and pulse were nearly 180 mmHg and 80 beats per minute with an average use of 5.5 antihypertensive medications per patient. After three months, mean blood pressure was reduced by an average of more than 20 mmHg. If anything, the reduction in blood pressure was enhanced after two and three years of follow-up, respectively [13]. After four years, systolic blood pressure was even reduced by an average of 53 mmHg (Fig. 5) [14]. The procedure of implantation proved to be safe. Placement of the electrode around the carotid sinus did not result in stenosis of the carotid artery. Patient satisfaction was very high, and patients reported better sleep quality, fewer headaches, and improved ability to concentrate.
A phase III trial (Rheos Pivotal Trial) with 265 patients has been initiated in the USA and Europe. This was a double-blind randomized placebo-controlled trial in patients with refractory hypertension. One month after implantation of the Rheos system, patients were randomized 2:1, i.e., in two thirds of patients the device was switched on immediately after randomization and in the remainder only after six months. Both groups of patients were followed up for twelve months after randomization. The study showed a reduction in mean systolic blood pressure of 35 mmHg after twelve months. More than 50% of patients achieved a target blood pressure value of ≤140 mmHg [15,16]. A slight initial decrease in glomerular filtration rate was observed after the device was turned on. This was interpreted as a response to blood pressure reduction. However, over the long-term, renal function remained stable [17].
In a subgroup analysis of the DEBuT-HT study, the effect of altered blood pressure on autonomic cardiac regulation was investigated in 21 patients [18]. A change in heart rate variability was noted, which correlated with sympathetic attenuation or parasympathetic activation with a corresponding reduction in blood pressure. In another study, BAT was shown to result in “reversed remodeling” of the left ventricle and atria, as well as improved arterial compliance [19]. In addition, improved functional performance of more than 37% (as measured by the 6-minute walk test) was observed in patients with symptomatic heart failure taking BAT. In addition, echocardiography revealed a significant reduction in left ventricular mass and posterior wall thickness as a sign of “reversed remodeling” of the left ventricle [20].
All of the above studies were performed with the first-generation blood pressure lowering device (Rheos). In a recent study with the second-generation implant (Barostim neo™), the same effect on blood pressure reduction was achieved as with the Rheos system [21].
Contrary to the fear that BAT could lead to a blunting of the baroreflex (“baroflex resetting”), a sustained dose-dependent reduction of blood pressure and heart rate could be observed in patients with refractory hypertension [22].
Study results to date have led to the inclusion of BAT as a treatment option in the ESH/ESC hypertension guidelines in 2013. It is recommended that BAT be considered in patients with refractory hypertension (recommendation class IIb, evidence level C). However, it was simultaneously recommended that the baroreceptor stimulator should be implanted only by experienced specialists and in selected hypertension treatment centers until more evidence on long-term efficacy and safety is available (class I, level C) [23].
The cost-effectiveness of BAT was analyzed in another study in patients suffering from refractory hypertension. At the same time, the impact of therapy on cardiovascular morbidity and mortality was also studied [24]. In this study conducted in Germany, the authors concluded that in patients with refractory hypertension, lifelong baroreceptor stimulation resulted in a 35% risk reduction for stroke, 19% risk reduction for myocardial infarction, and 23% risk reduction for renal failure requiring dialysis. On average, patients would live 1.66 years longer with a 2.17 increase in quality-adjusted life years (QALY). The cost of one QALY was estimated at 7797 euros. This value is much lower than the generally accepted cost-effectiveness threshold of 35 000 euros for a QALY [25]. It was concluded that BAT may be cost-effective compared with optimal hypertension drug treatment in patients with refractory hypertension.
BAT in heart failure patients
Because BAT not only lowers blood pressure but also has a beneficial effect on cardiac function, studies have been conducted in heart failure patients. Recently, a study of eleven heart failure patients (NYHA class III, EF <40%) in whom cardiac resynchronization therapy was not possible was published. Over six months, BAT was used in parallel with optimal drug therapy. The effectiveness of the treatment was measured by the change in muscle sympathetic nerve activity. In addition, clinical parameters for quality of life and functional performance were collected. Patients exhibited significant attenuation of sympathetic muscular nerve activity with significant improvements in baroreflex sensitivity, ejection fraction, NYHA class, quality of life, and 6-minute walk test. In addition, the number of rehospitalizations due to worsening heart failure was significantly reduced [26]. BAT proved to be compatible with implantable cardioverter defibrillator (ICD) and the two therapies appeared to have complementary effects [27].
In April 2013, a heart failure study was initiated including patients with a left ventricular ejection fraction of ≤35% and a NYHA Class III (Barostim Health Outcomes Prospective Evaluation for Heart Failure, Barostim HOPE4HF). This is a Phase II trial evaluating the efficacy and safety of the Barostim neo™ system in heart failure patients. This prospective randomized-controlled multicenter study will enroll 140 patients in the U.S. and Europe with the intent to compare Barostim neo™ therapy to standard heart failure therapy. Study endpoints are changes in ejection fraction, 6-minute walk test, NYHA classification, quality of life and renal function, and other parameters. The first results after six months are expected shortly.
Current situation
By early September 2014, a total of 420 Rheos devices and 380 Barostim neo™ stimulators had been implanted worldwide. Implantation proved to be safe and implantation risks are comparable to those of other implantable devices such as pacemakers or ICDs.
In Europe, Barostim neo™ is CE certified and available in Italy, Germany, Switzerland and Holland. Financial compensation is not yet universally regulated, but efforts are being made in this direction, knowing that BAT can be cost-effective in patients with refractory hypertension [24].
Summary
The carotid stimulator can be implanted with low risk and produces significant and sustained blood pressure reduction in patients with refractory hypertension. The hemodynamic efficiency is greater than expected and even sustained for more than five years. The aim of the treatment is to modulate the autonomic nervous system and thus dampen the sympathetic tone. In addition to blood pressure reduction, cardiac remodeling with decrease in left ventricular myocardial wall thickness is also observed. All these results have led to the integration of BAT in the 2013 ESH/ESC guidelines on arterial hypertension. The efficacy of this treatment in heart failure patients is currently being investigated in studies; however, positive effects have already been demonstrated in selected patient populations.
Literature:
- Fields LE, et al: The burden of adult hypertension in the United States 1999 to 2000: a rising tide. Hypertension 2004; 44: 398-404.
- Hajjar I, Kotchen TA: Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988-2000. JAMA : the journal of the American Medical Association 2003; 290: 199-206.
- Chobanian AV, et al: The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA : the journal of the American Medical Association 2003; 289: 2560-2572.
- Campbell DJ: Vaccination against high blood pressure. Current pharmaceutical design 2012; 18: 1005-1010.
- Wienemann H, et al: Treating resistant hypertension with new devices. Minerva cardioangiologica 2014; 62: 235-241.
- Zannad F, et al: Chronic vagal stimulation for the treatment of low ejection fraction heart failure: results of the neural cardiac therapy for heart failure (NECTAR-HF) randomized controlled trial. European heart journal 2014 [Epub ahead of print].
- Koch E: The reflex self-control of the circulation. Dresden, Steinkopff 1931.
- Muratori G: Histological observations on the structure of the carotid sinus in man and mammals. Oxford, Pergamon 1967.
- Brest AN, Wiener L, Bachrach B: Bilateral carotid sinus nerve stimulation in the treatment of hypertension. The American journal of cardiology 1972; 29: 821-825.
- Lohmeier TE, et al: Prolonged activation of the baroreflex produces sustained hypotension. Hypertension 2004; 43: 306-311.
- Scheffers IJM, et al: Sustained blood pressure reduction by baroreflex hypertension therapy with a chronically implanted system: 3-year data from the Rheos DEBuT study in patients with resistant hypertension. J Hypertens 2009; 27: S421.
- Schmidli J, et al: Acute device-based blood pressure reduction: electrical activation of the carotid baroreflex in patients undergoing elective carotid surgery. Vascular 2007; 15: 63-69.
- Scheffers IJ, et al: Novel baroreflex activation therapy in resistant hypertension: results of a European multi-center feasibility study. Journal of the American College of Cardiology 2010; 56: 1254-1258.
- Kroon AA, et al: Chronically implanted system: 4-year data of Rheos® DEBuT-HT study in patients with resistant hypertension. Journal of hypertension 2010; 28: 278.
- Bakris GL, et al: Baroreflex activation therapy provides durable benefit in patients with resistant hypertension: results of long-term follow-up in the Rheos Pivotal Trial. Journal of the American Society of Hypertension: JASH 2012; 6: 152-158.
- Bisognano JD, et al: Baroreflex activation therapy lowers blood pressure in patients with resistant hypertension: results from the double-blind, randomized, placebo-controlled rheos pivotal trial. Journal of the American College of Cardiology 2011; 58: 765-773.
- Alnima T, et al: Renal responses to long-term carotid baroreflex activation therapy in patients with drug-resistant hypertension. Hypertension 2013; 61: 1334-1339.
- Wustmann K, et al: Effects of chronic baroreceptor stimulation on the autonomic cardiovascular regulation in patients with drug-resistant arterial hypertension. Hypertension 2009; 54: 530-536.
- Bisognano JD, et al: Improved cardiac structure and function with chronic treatment using an implantable device in resistant hypertension: results from European and United States trials of the Rheos system. Journal of the American College of Cardiology 2011; 57: 1787-1788.
- Mohaupt MG, Schmidli J, Luft FC: Management of uncontrollable hypertension with a carotid sinus stimulation device. Hypertension 2007; 50: 825-828.
- Hoppe UC, et al: Minimally invasive system for baroreflex activation therapy chronically lowers blood pressure with pacemaker-like safety profile: results from the Barostim neo trial. Journal of the American Society of Hypertension: JASH 2012; 6: 270-276.
- Alnima T, et al: Sustained acute voltage-dependent blood pressure decrease with prolonged carotid baroreflex activation in therapy-resistant hypertension. Journal of hypertension 2012; 30: 1665-1670.
- Task Force for the Management of Arterial Hypertension of the European Society Of H, Task Force for the Management of Arterial Hypertension of the European Society Of C: 2013 ESH/ESC Guidelines for the Management of Arterial Hypertension. Blood pressure 2013; 22: 193-278.
- Borisenko O, et al: Cost-effectiveness of Barostim therapy for the treatment of resistant hypertension in European settings. Journal of hypertension 2014; 32: 681-692.
- Eichler HG, et al: Use of cost-effectiveness analysis in health-care resource allocation decision-making: how are cost-effectiveness thresholds expected to emerge? Value Health 2004; 7: 518-528.
- Gronda E, et al: Chronic baroreflex activation effects on sympathetic nerve traffic, baroreflex function, and cardiac haemodynamics in heart failure: a proof-of-concept study. European journal of heart failure 2014; 16: 977-983.
- Madershahian N, et al: Baroreflex activation therapy in patients with pre-existing implantable cardioverter-defibrillator: compatible, complementary therapies. Europace 2014; 16: 861-865.
CARDIOVASC 2014; 13(6): 6-11