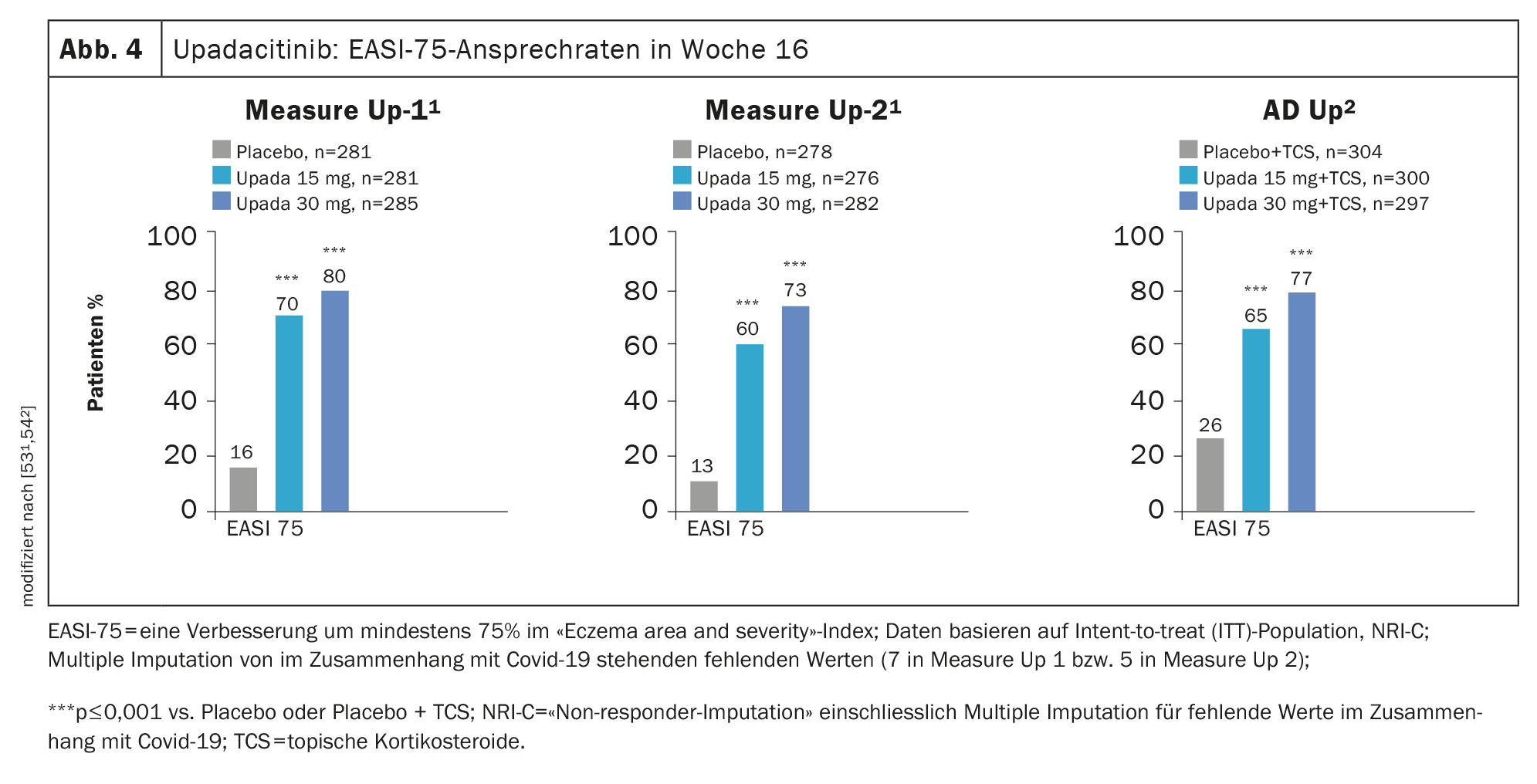New therapies in the form of specific antibodies and “small molecules” have ushered in a new era. Biologics intervene in the inflammatory process of atopic dermatitis by targeting individual cytokines. JAK inhibitors, on the other hand, are small-molecule agents that target inhibition of the JAK/STAT signaling pathway. In terms of personalized medicine, the focus is increasingly on selecting the most suitable drug for the individual.
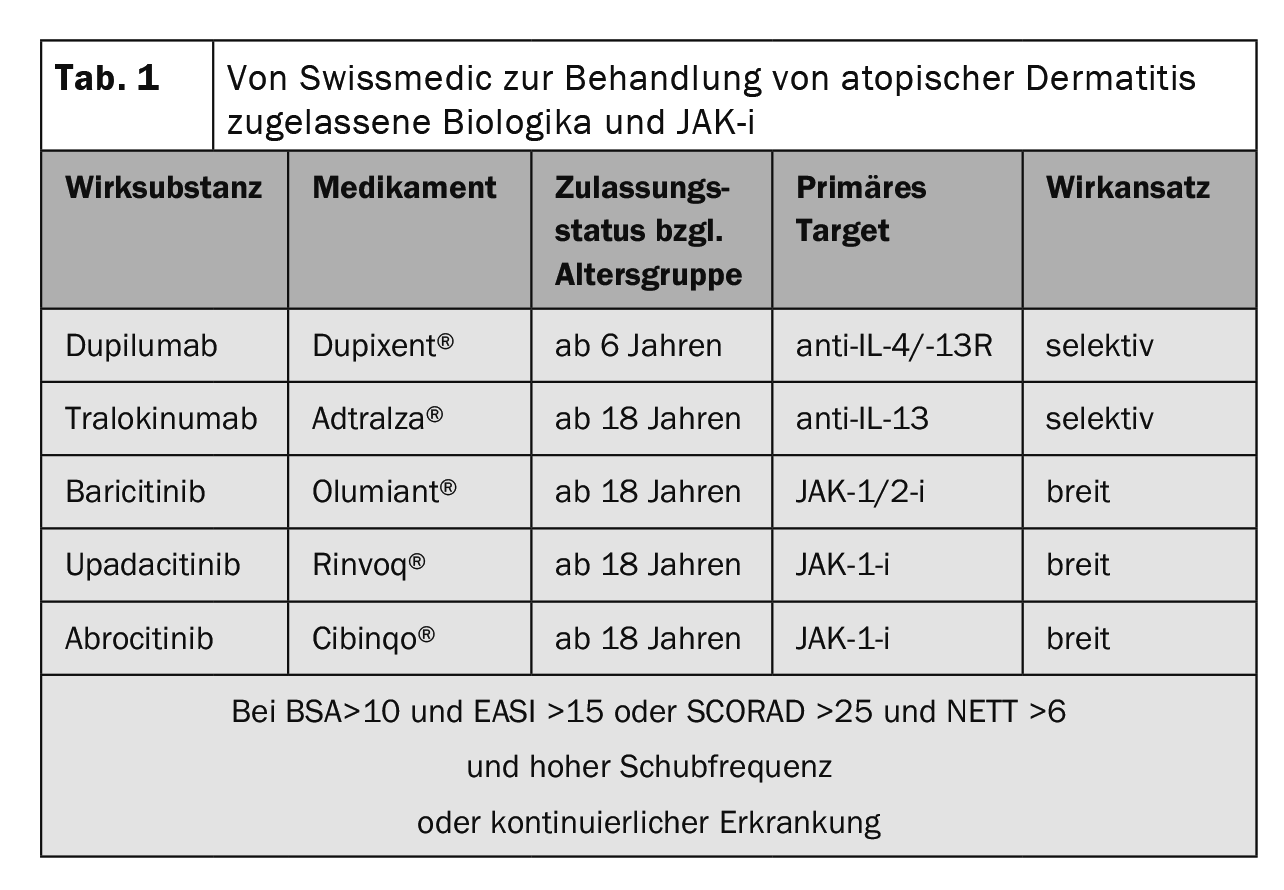
Genetic predisposition, environmental factors, and dysregulation of innate and adaptive immunity are involved in the complex pathophysiology of atopic dermatitis (AD). Type 2 immune responses not only represent the cellular correlate for skin inflammation in AD, but also significantly influence barrier dysfunction and microbial dysbiosis [1,2]. The Severity Scoring of Atopic Dermatitis (SCORAD) can be used to define the severity of AD. Below 25 points, atopic eczema is classified as mild, between 25 and 60 points as moderate, and above 60 as severe [3,4]. In about one-fifth of AD cases, the courses are moderate to severe. The overall therapeutic goals include reduction of pruritus as well as improvement of the inflammatory response and restoration of an intact skin barrier [5]. International guidelines recommend that the treatment of AD be individually adapted to the different phases, severity, and chronicity according to a stepwise therapy regimen [6]. In recent years, there has been significant progress, particularly in the area of systemic treatment options [7]. In addition to the monoclonal antibodies tralokinumab and dupilumab administered as subcutaneous injections, the Janus kinase (JAK) inhibitors baricitinib, upadacitinib, and abrocitinib are available in oral dosage form (Table 1) .
Currently approved biologics – IL-4 and IL-13 as key cytokines.
The Th2 cytokines interleukin(IL)-4 and IL-13 are significantly involved in IgE-mediated allergic responses and play a key pathophysiological role in the inflammatory responses and barrier dysfunction in AD [10,14,25]. A positive feedback loop results in decreased keratinocyte differentiation and increased epidermal hyperplasia.
Dupilumab specifically blocks IL-4/IL-13 signaling pathways and is the first monoclonal antibody approved for the treatment of atopic dermatitis. In Switzerland, dupilumab (Dupixent®) has been approved for the treatment of moderate to severe atopic dermatitis in adults since 2019, followed later by indication expansion for adolescents, and since June 2022, dupilumab can also be used for children aged 6 years and older [24].
In adults, the recommended starting dose is 600 mg dupilumab (two 300 mg injections) and the maintenance dose is 300 mg every two weeks. For children and adolescents from 6 to 17 years, the dosage recommendation depends on body weight.
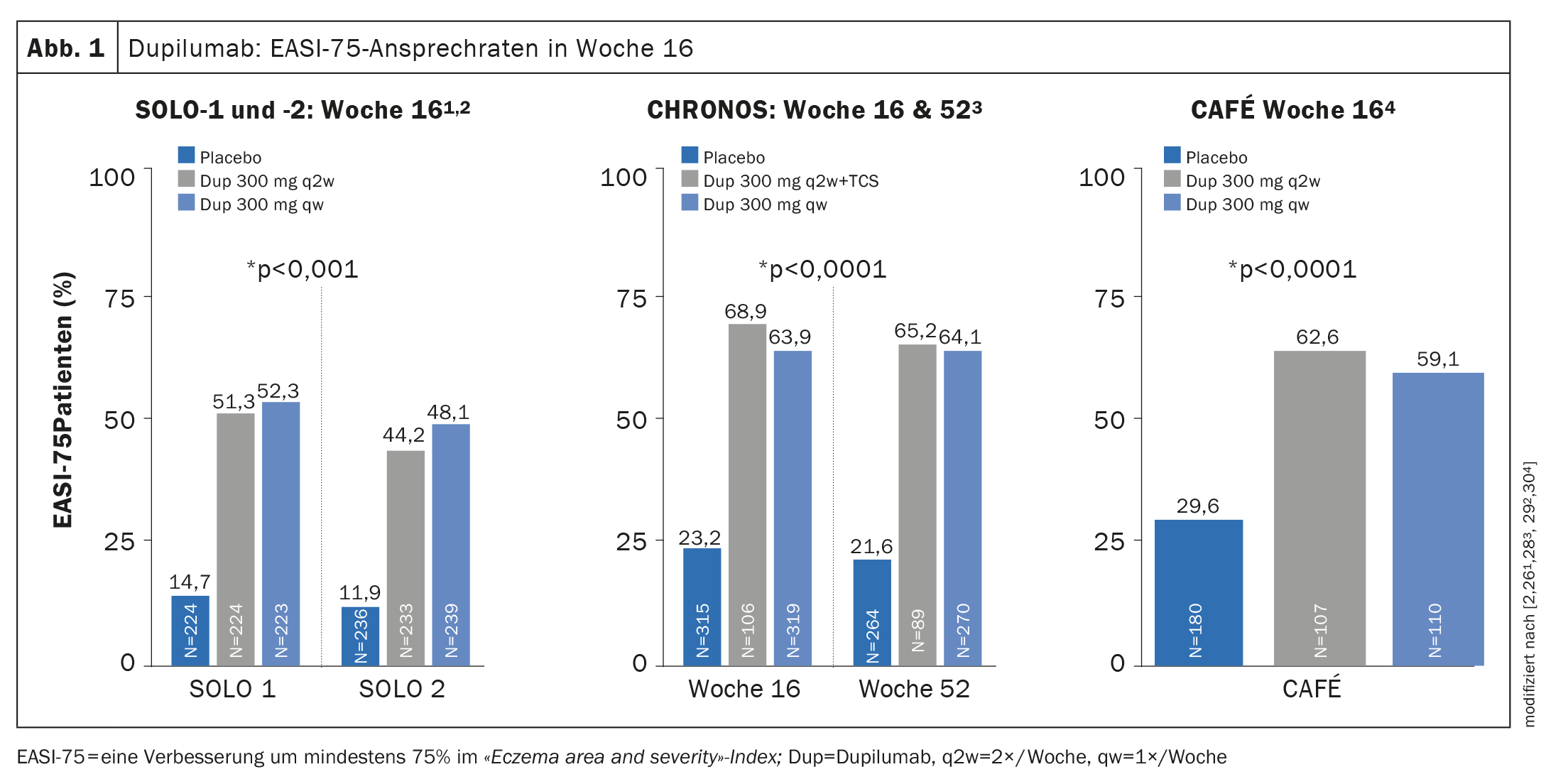
Evidence of efficacy in phase III trials: in both SOLO-1 and -2 (monotherapy) and CHRONOS (combination therapy with concomitant TCS), more than 35% of patients achieved an Investigator’s Global Assessment (IGA) score of 0 or 1 at week 16, corresponding to appearance-free or near-appearance-free skin [26,28]. The EASI-75 response rate was achieved in both SOLO-1 and -2 and in CAFÉ at week 16 (Fig. 1) and in CHRONOS additionally also at week 52 [26,28–30]. In the OLE trial, the IGA 0/1 response rate reached a plateau of approximately 60% at week 48, which was maintained until the study endpoint at week 76 [31,43].
In addition, dupilumab showed rapid relief of pruritus in a placebo comparison with an improvement in least square means of approximately 20% within two weeks of treatment initiation [26,28–31].
The approval of dupilumab for adolescents is based on clinical data from the LIBERTY AD program. The efficacy and safety of monotherapy with dupilumab at a dose of 200 mg or 300 mg every two weeks were assessed in a randomized, double-blind, controlled trial in 251 patients 12-17 years of age with moderate-to-severe AD [32].
With dupilumab, 42% achieved an EASI-75 response and 24% achieved IGA 0/1. In the placebo group, the corresponding values were 8% and 2%, respectively. For the pediatric population of 6-11-year-olds, the efficacy and safety of dupilumab in combination with topical corticosteroids (TCS) were evaluated in the AD-1652 and AD-1434 studies [33].
Safety profile: the most common side effects of dupilumab include injection site reactions and conjunctivitis, and joint pain, oral herpes, and eosinophilia are also reported [33]. Conjunctivitis can be successfully treated in most cases with topical medications (hyaluronic acid or fluorometholone (0.1%) eye drops or tacrolimus (0.03) eye ointment) [34]. The safety signals of dupilumab in children and adolescents were found to be broadly comparable to those in adults.
Contraindications: Except for very rare general systemic hypersensitivity reactions, no contraindications are known [24].
Therapy Monitoring and Precautions: It is recommended that patients’ vaccination status be updated according to current vaccination recommendations prior to initiation of treatment with dupilumab [24]. Live vaccines and live attenuated vaccines must not be used concurrently with dupilumab because clinical safety and efficacy have not been established.
Tralokinumab is a human monoclonal antibody that specifically neutralizes IL-13. In Switzerland, tralokinumab (Adtralza®) has been approved since June 2022 for adult patients with moderate to severe AD when therapy with topical drugs is not effective [24].
The initial dose for adults is 600 mg (4 injections of 150 mg each), followed by a maintenance dose of 300 mg (2 injections of 150 mg each), with a dosing interval of 2 weeks each. From week 16, the interval can be extended to 4 weeks.
Unlike dupilumab, tralokinumab does not bind to the receptor but to the soluble part of IL-13, preventing interaction with the IL-13Rα1 receptor [35,36]. IL-13 is a key cytokine in the pathogenesis of atopic dermatitis [35]. Overexpression of IL-13 has been found in both lesional and non-lesional atopic skin and, moreover, IL-13 levels have been found to correlate with AD severity [37].
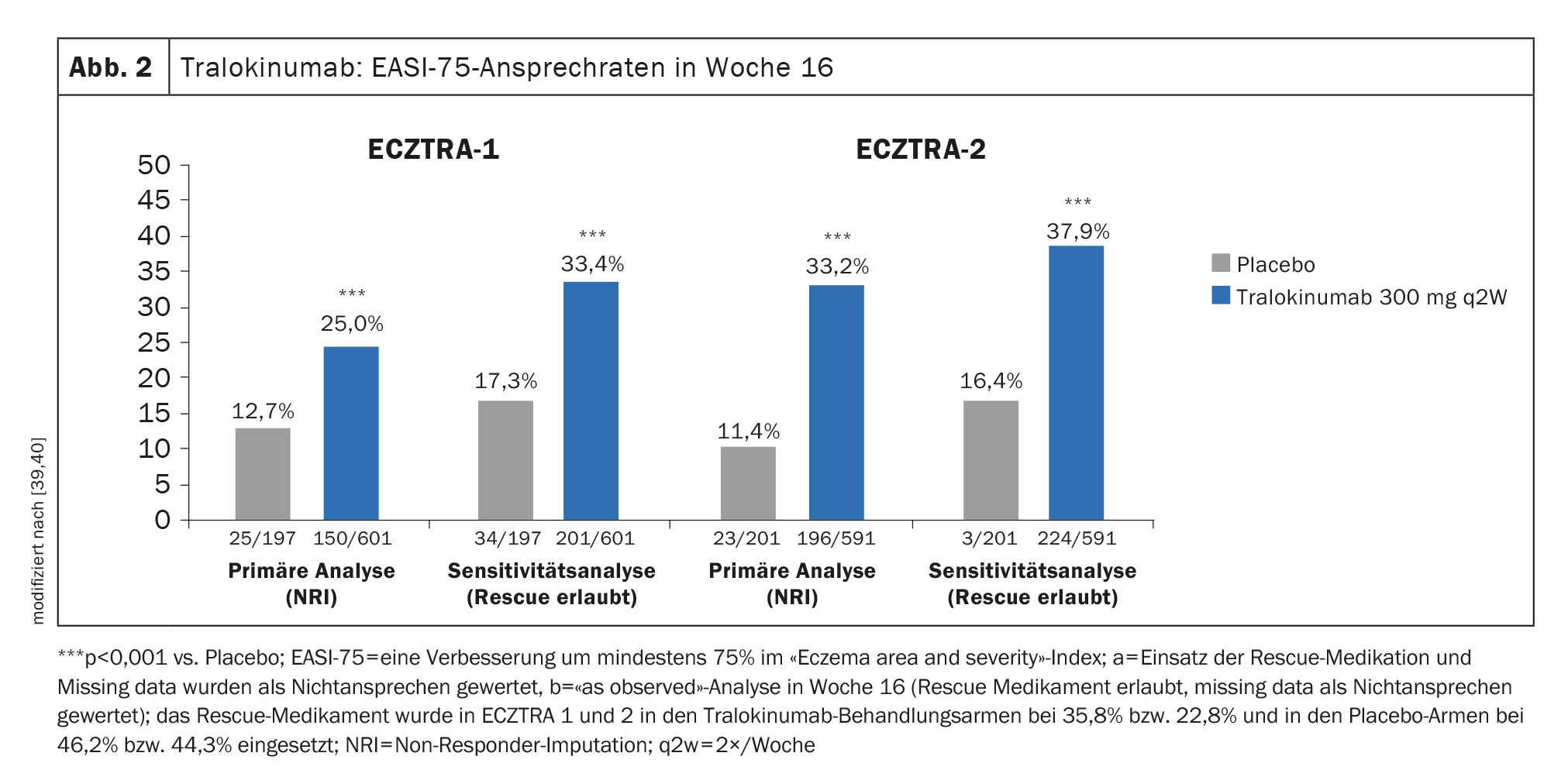
Evidence of efficacy in phase III trials: in ECZTRA-1 and -2, 15.8% and 22.2%, respectively, of patients treated every two weeks (q2w) with tralokinumab 300 mg achieved IGA 0/1 (lesion-free or nearly lesion-free) at week 16, compared with only 7.1% and 10.9%, respectively, in the placebo comparison group [39,40]. EASI-75 was achieved by 25% of tralokinumab 300 mg q2w-treated study participants at week 16 in ECZTRA-1, and the corresponding proportion was 33.2% in ECZTRA-2 (Fig. 2) [39,40].
In ECZTRA-3, significantly more study participants achieved EASI-75 at week 16 with tralokinumab 300 mg q2w plus TCS, 56.0%, than with placebo, where this rate was 35.7%. Regarding IGA 0/1, the tralokinumab arm also proved to be significantly superior in the placebo comparison (38.9% vs. 26.2%) [37].
Safety Profile: The most common adverse reactions are upper respiratory tract infection/cold and injection site reactions. Conjunctivitis occurred slightly less frequently than with dupilumab [41].
Contraindications: Except for very rare general systemic hypersensitivity reactions, no contraindications are known [24].
Therapeutic Monitioring and Precautions: Live vaccines and live attenuated vaccines should not be administered concurrently with tralokinumab because clinical safety and efficacy have not been established. Refer to current vaccination recommendations for time intervals between live vaccination and treatment with tralokinumab.
JAK inhibitors are also on the rise
Inhibitors of Janus kinase (JAK) suppress the intracellular cytokine response (JAK-STAT mechanism). The mechanism of action of JAK inhibitors is to use “small molecules” to achieve blockade of the JAKs on which cytokine receptors rely. With baricitinib, upadacitinib and abrocitinib, three orally administrable JAK-i are currently approved in Switzerland for the systemic treatment of moderate to severe AD [24]. Pathogenetically central cytokines that initiate their cytokine response via the JAK-STAT pathway are IL-4, IL-13, and IL-31 [44].
What is particularly interesting about JAK-i as a therapeutic option is its rapid onset of action. The side effect profile of JAK-i is compound- and dose-dependent, with infections occurring more frequently compared to biologics and certain laboratory parameters being altered during therapy, necessitating intensified patient monitoring.
Baricitinib is an oral selective JAK1 and JAK2 inhibitor. Through JAK1, the signals of key adaptive and innate cytokines IL-4, IL-13, IL-22, IL-31, TSLP, IFN-γ, and IL-6 are transmitted, and JAK2 is necessary for the function of the IL-5 receptor, which is required in the recruitment of eosinophilic granulocytes [1].
Baricitinib (Olumiant®) has been approved in Switzerland since February 2021 for the treatment of adult patients with moderate to severe atopic dermatitis [24,45]. The standard dosage is 4 mg (1×/d p.o.). The 2 mg dose (1×/d p.o.) is considered in patients 75 years of age or older or patients with chronic/recurrent infections or a creatinine clearance of 30-60 ml/min. If no therapeutic benefit of baricitinib is evident after 8 weeks, discontinuation of therapy should be considered.
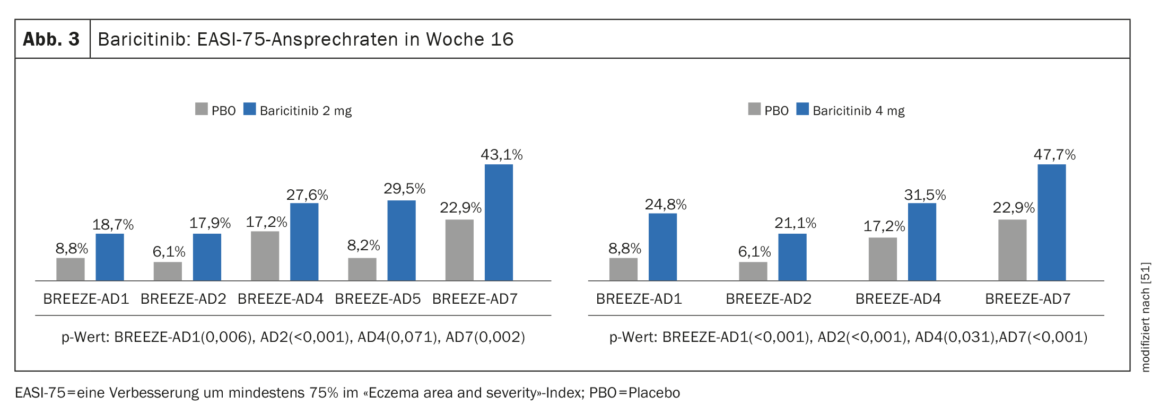
Evidence of efficacy in phase III studies: In addition to BREEZE-AD1 and BREEZE-AD2, in which baricitinib monotherapy resulted in significant symptom reduction, the pivotal phase III study program included the real-world study BREEZE-AD7 to evaluate efficacy and safety of baricitinib in combination with topical corticosteroids (TCS) [45–47]. 329 adult patients with moderate-to-severe AD were randomized in BREEZE-AD7 in a 1:1:1 ratio to baricitinib 4 mg plus TCS or baricitinib 2 mg plus TCS or placebo. At week 16, approximately 47.7% of patients on therapy with baricitinib 4 mg in combination with TCS showed an EASI-75 response, compared with 22.9% in the placebo group (p<0,001) (Fig. 3). In the 2 mg baricitinib arm, the corresponding values were 43.1% and 22.9%, respectively. (Fig. 3).
An improvement in itch by ≥4 points on the numerical rating scale (NRS) within the first 16 weeks was achieved in 44% of patients treated with baricitinib (4 mg plus TCS), compared to 20% with placebo plus TCS (p<0.01) [48]. Comprehensive trial data are available on the tolerability of baricitinib in AD from a total of 8 randomized trials involving 2531 patients and 2247 patient-years [49].
Safety Profile: The most commonly reported adverse reactions during therapy with baricitinib are increased LDL cholesterol, upper respiratory tract infections, headache, herpes simplex, and urinary tract infections [50]. The incidence of serious infections with baricitinib was similar to placebo.
Contraindications: Baricitinib is contraindicated during pregnancy. Women of childbearing potential must use a reliable method of contraception during the use of baricitinib and for at least one additional week after discontinuation of treatment In patients with severe hepatic impairment or creatinine clearance <30 ml/min, the use of baricitinib is not recommended [50].
Therapy Monitoring and Precautions: Patients should be evaluated for severe hepatic and renal dysfunction and history of risk factors for deep vein thrombosis or pulmonary embolism prior to initiation of treatment. Lipid levels (total cholesterol, HDL, LDL, triglycerides) should also be collected and monitored 12 weeks later or according to the hyperlipidemia guideline. In addition, patients must be tested for tuberculosis (TB) before starting baricitinib therapy. Baricitinib should not be used in active TB, and in previously untreated latent TB, anti-TB therapy should be considered before initiating baricitinib treatment. If a patient develops a herpes zoster infection, treatment with baricitinib should be temporarily interrupted until the infection has resolved. Screening for viral hepatitis should be performed before starting baricitinib therapy. Patients with proven active hepatitis B or hepatitis C infection were excluded from the clinical trials. It is recommended in advance of baricitinib therapy to bring all immunizations up to date in accordance with current immunization recommendations. The use of live attenuated vaccines is not recommended during or immediately prior to baricitinib treatment.
Upadacitinib is a perorally bioavailable, selective and reversible Janus kinase inhibitor that primarily inhibits JAK1. In Switzerland, upadacitinib (Rinvoq®) has been approved for the treatment of moderate-to-severe AD in adults since November 2021 [52]. The recommended oral dose of is 15 mg upadacitinib once daily.
Evidence of efficacy in phase III studies: The efficacy of upadacitinib in AD was evaluated in the phase III MEASURE Up-1, MEASURE Up-2, and AD Up studies, with a total of 2584 patients included in the study program. In all three studies, study participants received upadacitinib 15 mg or 30 mg once daily or placebo for a period of 16 weeks and significant improvement in SCORAD and EASI scores was demonstrated in the placebo comparison (Fig. 4). Also, a faster improvement in the appearance of the skin and itching was achieved [52].
In MEASURE Up-1 and -2, upadacitinib was studied as monotherapy in moderate-to-severe AD in a subject population totaling 1683 patients. After 16 weeks, more than 40% on upadacitinib 15 mg (1×/d) achieved an EASI 90 response and significant relief of pruritus, whereas in the placebo groups both were seen in only about one in ten patients [53,54]. In an integrated analysis of both the Measure Up-1 and Measure-2 trials, at week 52, upadacitinib showed a 62% EASI-90 response and 65% significant improvement in pruritus . [55]. An EASI-75 response was achieved by approximately 80% [55]. In addition, treatment with upadacitinib was associated with a rapid response: improvement in pruritus was observed after only 2 days .
Safety profile: the side effect profile of upadacitinib is similar to other JAK inhibitors. The most common are upper respiratory tract infections and acne; less common are herpes simplex, headache, and elevated creatine phosphokinase in the blood [52].
Contraindications: Upadacitinb is contraindicated during pregnancy; women of childbearing potential must use reliable contraceptive methods both during treatment and up to four weeks after the last dose of upadacitinib. It is recommended that upadacitinib not be used in patients with an absolute lymphocyte count of less than 500 cells/mm3, an absolute neutrophil count of less than 1000 cells/mm3 , or a hemoglobin level of less than 8 g/dL [24].
No dose adjustment is required in patients with mild or moderate hepatic impairment. In cases of severe hepatic impairment (Child-Pugh C), the use of upadacitinib is not recommended. Renal insufficiency has no clinically relevant effect on upadacitinib exposure [52].
Therapy Monitoring and Precautions: Tuberculosis (TB) screening should be performed prior to initiation of therapy with upadacitinib. Upadacitinib must not be used in patients with active TB. In patients with untreated latent TB, consider anti-TB therapy before initiating treatment with upadacitinib. If herpes zoster occurs in a patient, consideration should be given to interrupting treatment with upadacitinib until the infection resolves. Screening for viral hepatitis and monitoring for possible reactivation should be performed prior to initiation of and during therapy with upadacitinib. Patients testing positive for hepatitis C antibody and hepatitis C virus RNA were excluded from the clinical trials.
Prior to initiation of therapy with upadacitinib, it is recommended that patients’ immunization status be reviewed according to current immunization guidelines and that all required immunizations be obtained. The use of live attenuated vaccines during or immediately before treatment with upadacitinib is not recommended [52].
Abrocitinib is an oral selective JAK-1 inhibitor. In Switzerland, abrocitinib (Cibinqo®) has been approved for the treatment of atopic dermatitis in adults since April 2022 [24].
Evidence of efficacy in phase III studies: The efficacy and safety of abrocitinib as monotherapy and in combination with active topically applied background therapies over a 12- to 16-week period were evaluated in 1616 patients in the placebo-controlled phase III MONO-1, MONO-2, and COMPARE studies. In the REGIMEN randomized-controlled trial, which is also pivotal, abrocitinib was studied over a 52-week study period in a total of 1233 study participants [56].
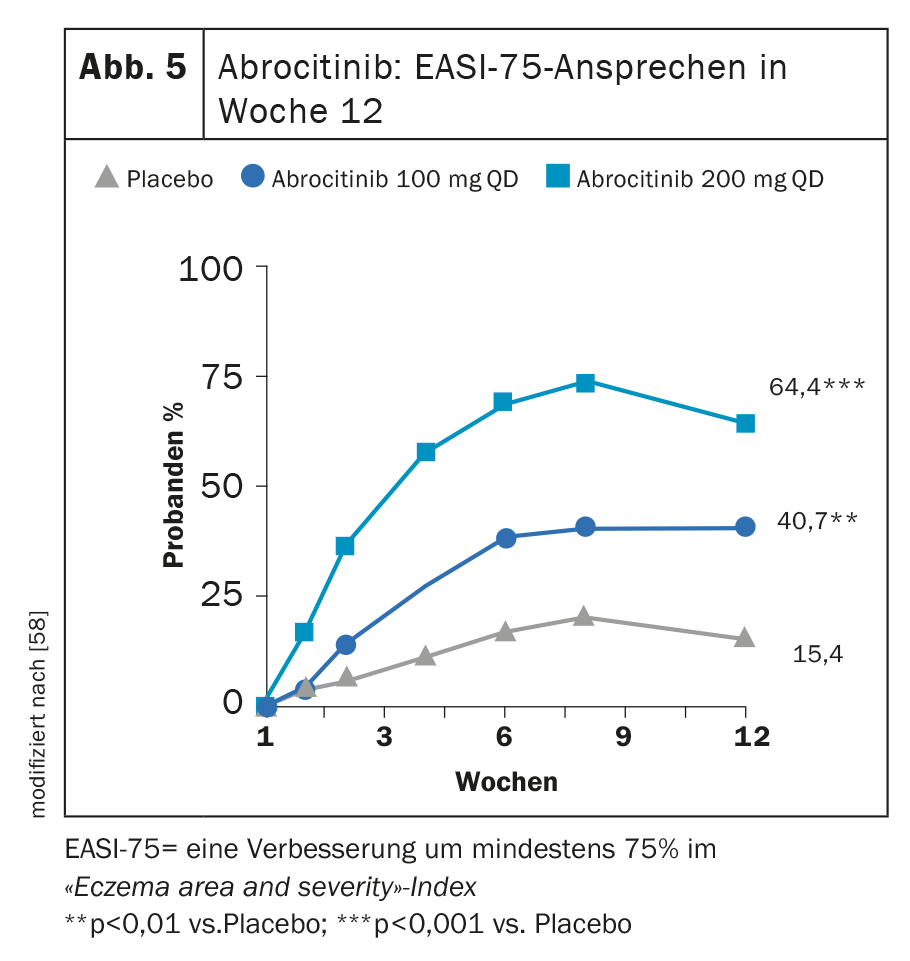
In MONO-1 and MONO-2, treatment with abrocitinib 100 mg (1× d) achieved both primary endpoints IGA 0 or 1 and/or EASI-75 in a significantly greater proportion at week 12 (Fig. 5) [24]. In combination with TCS, the JAK inhibitor proved significantly superior to placebo in the COMPARE trial at week 16 with respect to these two endpoints. A reduction in itch by ≥4 points on the pruritus NRS manifested with abrocitinib versus placebo as early as week 2 in a significantly higher proportion of study participants. [57]. At the high dose of 200 mg, 43.8% of patients showed improvement in IGA and 64.6% showed an EASI-75 [58] at 12 weeks in a phase IIb study.
Safety Profile: The most commonly reported adverse reactions are nausea, headache, acne, herpes simplex, creatine phosphokinase elevated in blood, vomiting, dizziness, and upper abdominal pain. The most common serious side effects (0.3%) are infections [56].
Contraindications: Abrocitinib is contraindicated during pregnancy. Women of childbearing potential should be advised to use effective contraception during treatment and for one month after the end of peroral use of upadacitinib. Abrocitinib is contraindicated in severe hepatic impairment (Child-Pugh class C). No dose adjustment is required in cases of mild renal impairment. In cases of moderately severe renal impairment (eGFR 30 ml/min to <60 ml/min or eGFR <30 ml/min), dose reduction should be performed according to the drug’s SmPC [56].
Therapy Monitoring and Precautions: Patients should be tested for tuberculosis (TB) before starting abrocitinib treatment. Abrocitinib must not be given to patients with active TB. In patients with untreated latent TB, preventive therapy for latent TB should be started before initiating treatment.
If a patient develops a herpes zoster infection, consideration should be given to temporarily interrupting treatment with abrocitinib until the infection has resolved. Screening for viral hepatitis should be performed prior to initiation and during therapy according to clinical guidelines. Patients with proven active hepatitis B or hepatitis C infection were excluded from the clinical trials. In addition, it is recommended that all immunizations be brought up to date in accordance with current immunization recommendations prior to initiation of abrocitinib treatment. The use of live attenuated vaccines should be avoided during or immediately before abrocitinib treatment [56].
Further promising active ingredients are in the pipeline
Other agents currently being researched for use in AD include [2]:
- Lebrikizumab: monoclonal antibody that specifically binds to freely circulating IL-13. In addition to the two phase III monotherapy trials ADvocate 1 and 2, ADhere is investigating the combination of lebrikizumab with TCS [62].
- Nemolizumab: monoclonal antibody that blocks the α-subunit of the IL-31 receptor. Study data to date demonstrate strong itch relief in particular [63]. In a 16-week randomized trial with 2:1 randomization, at week 16, the nemolizumab group (n=143) showed a mean percent change in VAS score of -42.8% versus -21.4% on placebo (n=72) [64].
- Tezepelumab: antibody against TSLP (thymic stromal lymphoprotein). TSLP is an epithelial cell-derived cytokine that is particularly associated with pruritus in AD [65]. In the Phase IIa ALLEVIAD study, at week 12, the mean percent improvement in peak pruritus NRS tezelumab plus TCS vs. placebo was 33.54 (SD 6.02) vs. 25.41 (SD 6.06); p=0.258) [68].
- anti-Ox-40 antibody: Ox-40 is a costimulatory receptor on activated T cells [65]. In a randomized, double-blind, placebo-controlled phase IIa study, administration of the anti Ox-40 antibody at 4-week intervals resulted in significant clinical improvements by day 71 [70].
- Brepocitinib: topical TYK2/JAK1 inhibitor.
- Aryl hydrocarbon receptor modulators: cytoplasmic receptor system affecting inflammatory mechanism and barrier molecules.
Take-Home Messages
- New therapies in the form of specific antibodies and so-called small molecules have ushered in a new era. Interleukin (IL-)4 and IL-13 are the two major pro-inflammatory cytokines in AD. In addition, there are other inflammatory mediators that play a role.
- Biologics interfere with the inflammatory process in AD by targeting individual cytokines. JAK inhibitors, on the other hand, are small-molecule agents that target inhibition of the JAK/STAT signaling pathway.
- When a cytokine binds to the extracellular domain of its receptor, Janus kinases activate intracellular transcription factors that control the reading of a variety of genes in the nucleus [1].
- The most significant differences among highly effective modern systemic therapeutics relate to dosage form, frequency of use, side effect profile, and therapy monitoring. In the sense of personalized medicine, the aim is increasingly to select the drug that best suits disease characteristics and other person- and context-related features.
Literature:
- Lauffer F, Biedermann T: Dtsch Arztebl 2021; 118(24): [24]; DOI: 10.3238/PersDerma.2021.06.18.04
- “Atopic dermatitis, what’s new?”, Univ.-Prof. Dr. Paul-Gunther Sator, DermAlpin Salzburg, 30-31.10.2021.
- Deutsche Haut- und Allergiehilfe e.V, www.dha-neurodermitis.de/therapie/grundlagen-der-therapie.html,(last accessed, Jan. 06, 2023).
- Severity scoring of atopic dermatitis: the SCORAD index. Dermatology 1993; 186(1): 23-31.
- Nygaard U, Deleuran M, Vestergaard C: Dermatology 2017; 233: 333-343.
- AWMF: Guideline Neurodermatitis, Register number 013-027, Classification S2k Status: 31.03.2015.
- DFP Literature Study: Atopic Dermatitis, www.oeadf.at/files/E-Learning/ClinicumDerma_12_032017.pdf,(last accessed Jan. 06 , 2023).
- .Leung DYM, Guttman-Yassky E: J Allergy Clin Immunol 2014; 134: 769-779.
- Kim BE, Leung DY, Boguniewicz M, Howell MDl: Clin Immunol 2008; 126: 332-337.
- Akdis CA, et al: Allergy 2020; 75(7): 1582-1605.
- Hoffjan S, Stemmer S: Arch Dermatol Res 2015; 307: 659-670.
- Darsow U, et al: J Eur Acad Dermatol Venereol 2010; 24: 317-328.
- Williams MR, Gallo RL: Curr Allergy Asthma Rep 2015; 15: 65.
- Nguyen JK, et al: Arch Dermatol Res 2020; 312(2): 81-92.
- Weidinger S, et al: Nat Rev Dis Primers 2018; 4(1): 2.
- Clark JD, et al: J Med Chem 2014; 57: 5023-5038.
- Howell MD, et al: Front Immunol 2019; 10: 2342.
- Chiesa Fuxench ZC, Ong P: Poster presented at AAD 2018. Poster 6236.
- Drucker AM, et al: J Invest Dermatol 2017; 137(1): 26-30.
- Ronnstad ATM, et al: JAAD 2018; 79: 448-456.
- Reich K, et al: International Society of Atopic Dermatitis 2021.
- Brunner PM, et al: J Invest Dermatol 2017; 137: 18-25.
- Silverberg JI: Ann Allergy Asthma Immunol 2019; 123(2): 144-151.
- Technical information, www.swissmedicinfo.ch, (last accessed 06.01.2022).
- 25 Silverberg J: Clin Dermatol 2017; 35: 360-366.
- Simpson EL, et al: N Engl J Med 2016; 375: 2335-2348.
- Paller AS et al: J Am Acad Dermatol. 2016;75(3): 494-503.
- Blauvelt A, et al: The Lancet 2017; 389(10086): 2287-2303.
- Simpson EL, et al. Late-breaking abstract, EADV 2016 abstract D3T01.1C.
- de Bruin-Weller, M. et al: Br J Dermatol 2018; 178(5): 1083-1101.
- Deleuran M et al: J Am Acad Dermatol 2020; 82(2): 377-388.
- Simpson EL, et al: JAMA Dermatol 2020; 156(1): 44-56.
- European Medicines Agency: Dupilumab, Product Information, https://ec.europa.eu/healthhttps://ec.europa.eu/health, last accessed 06.01.2023).
- Wollenberg A, et al: J Allergy Clin Immunol Pract. 2018 Sep-Oct; 6(5): 1778-1780.e1.
- Schmid-Grendelmeier P: Dermatology Practice 2021; 31(4): 10-14.
- Wollenberg A, et al: JDDG 2021; 19(10): 1435-1442.
- Silverberg JI, et al: Br J Dermatol 2021; 184: 450-463.
- Petronelli M: Tralokinumab meets endpoints for all phase 3 studies, Dermatology Times, Dec 16, 2019, www.dermatologytimes.com, (last accessed Jan 06, 2023).
- Simpson E, et al: Presentation at AAD VMX, June 12-14 and on-demand, 2020, USA.
- Wollenberg A, et al: Br J Dermatol 2021; 184(3): 437-449.
- European Medicines Agency, Tralokinumab, Product Information,https://ec.europa.eu/healthhttps://ec.europa.eu/health, last accessed Jan. 06, 2023).
- “World Neurodermatitis Day on September 14 Neurodermatitis: Systemically acting therapeutics improve treatment,” Berufsverband der Deutschen Dermatologen e.V. (BVVD), Berlin Sept. 03, 2021.
- Futamura MJ: Am Acad Dermatol 2016; 74: 288-294.
- Klein B, Treudler R, Simon JC: JDDG 2022; 20(1): 19-25.
- Simpson EL, et al. Presented at:24th World Congress of Dermatology; June 10-15, 2019; Milan, Italy.
- Melo A, Carrascosa JM, Torres T: J Dermatolog Treat 2021 Aug 23: 1-10. Epub ahead of print.
- Wollenberg A, et al: JEADV 2021; 35(7): 1543-1552.
- Reich K, et al: JAMA Dermatol 2020; 156(12): 1333-1343.
- Silverberg JI, et al: JAMA Dermatol 2021, 157: 691-699.
- European Medicines Agency: Baricitinib, Product Information, https://ec.europa.eu/health, last accessed 06.01.2023).
- 51. radi G, et al: Healthcare (Basel) 2021 Nov 18; 9(11):1575. healthcare-09-01575-v2.pdf.
- European Medicines Agency: Upadacitinib, Product Information, https://ec.europa.eu/health, last accessed 06.01.2023).
- Guttman-Yassky E, et al: Lancet 2021; 397(10290): 2151-2168.
- Reich K, et al: Lancet 2021; 397(10290): 2169-2181.
- Simpson EL, et al: Presentation at the 2021 Dermatology Education Foundation (DEF) Essential Resource Meeting (DERM2021), August 5-8, 2021, Las Vegas NV.
- European Medicines Agency: Abrocitinib, Product Information, https://ec.europa.eu/health/, (last accessed Jan. 06, 2023).
- “Atopic dermatitis: the JAK/STAT pathway as a therapeutic target ” https://medizinonline.com/der-jakstat-signalweg-als-therapeutische-zielstruktur,(last accessed Feb 16, 2023).
- Gooderham MJ, et al: JAMA Dermatol 2019; 155(12): 1371-1379.
- Guttman-Yassky E, et al: JAMA Dermatol 2020; 156(4): 411-420.
- Moyle M, et al: Exp Dermatol 2019; 28(7): 756-768.
- 61. Loh TY, et al: J Asthma Allergy 2020; 13: 109-114.
- 62. “Eli Lilly Submits BLA for Lebrikizumab AD Treatment.”
www.dermatologytimes.com/view/eli-lilly-submits-bla-for-lebrikizumab-ad-treatment,(last accessed 06.01.2023) - Ruzicka T, et al: N Engl J Med 2017; 376: 826-835.
- Kabashima K, et al: N Engl J Med 2020; 383: 141-150.
- 65 Quint T, Bangert C: hautnah 2021; 20: 37-44.
- 66 Ziegler SF, et al: Adv Pharmacol 2013; 66: 129-155.
- Shikotra A, et al: J Allergy Clin Immunol 2012; 129: 104-111.e1-9.
- Simpson EL, et al: J Am Acad Dermatol 2019; 80: 1013-1021.
- Suga H, Sato S: Novel topical and systemic therapies in atopic dermatitis. Immunol Med 2019; 42(2): 84-93.
- Guttman-Yassky E, et al: J Allergy Clin Immunol 2019; 144(2): 482-493.e487.
- Freund Y, et al: FEBS Lett 2012; 586: 3410-3414.
- Dong C, et al: J Pharmacol Exp Ther 2016; 358: 413-422.
- Jarnagin K, et al: J Drugs Dermatol 2016; 15(4): 390-396.
- Zane LT, et al: Pediatr Dermatol 2016: 33(4): 380-387.
DERMATOLOGIE PRAXIS 2023; 30(1): 5–11