Merkel cell carcinoma is a rare but aggressive form of skin cancer. It mainly affects older people, with the median age at diagnosis being 75 years. Adjuvant radiotherapy is recommended after surgical excision of the primary tumor. In advanced stage or distant metastases, immunotherapy should be considered. The only PD-L1 antibody approved in Switzerland to date has revolutionized treatment options.
“Merkel cell carcinoma is extremely heterogeneous in clinical presentation,” reported Anja Wysocki, MD, co-chief of dermatology at Luzerner Kantonsspital [1]. In addition to the nodular form, which is most common, plaque-like variants rarely occur; these are found primarily on the trunk and proximal extremities.
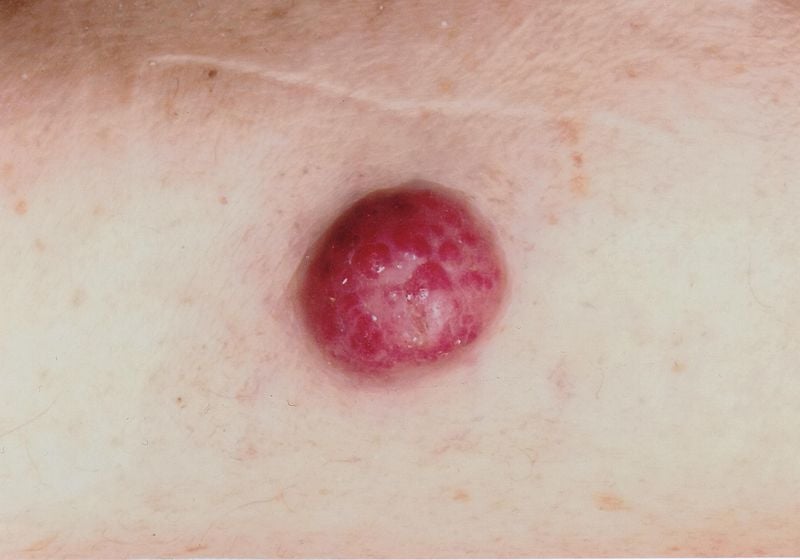
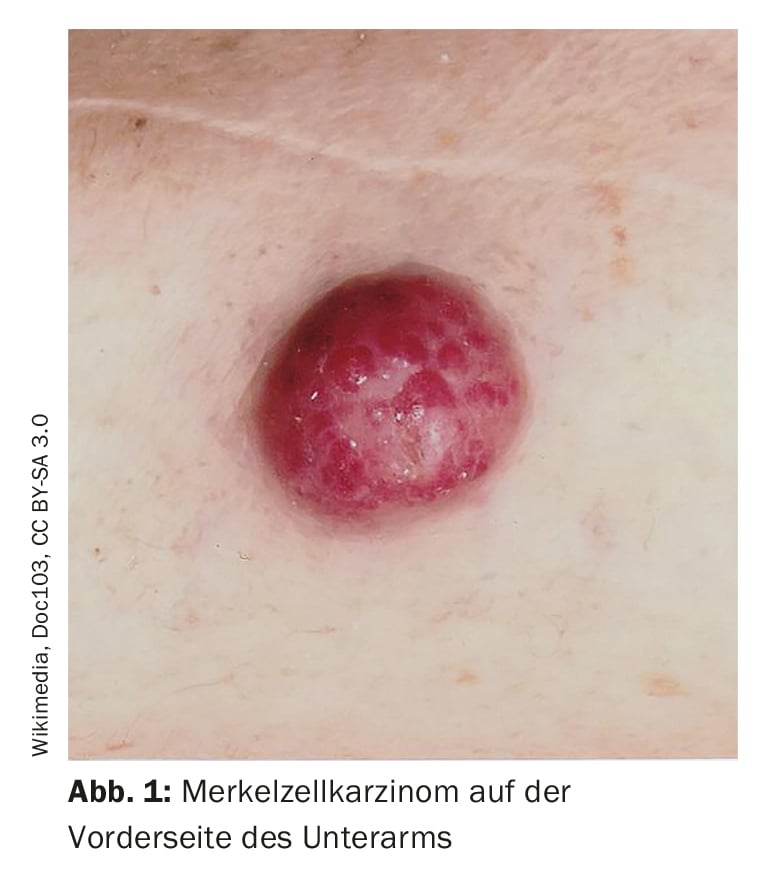
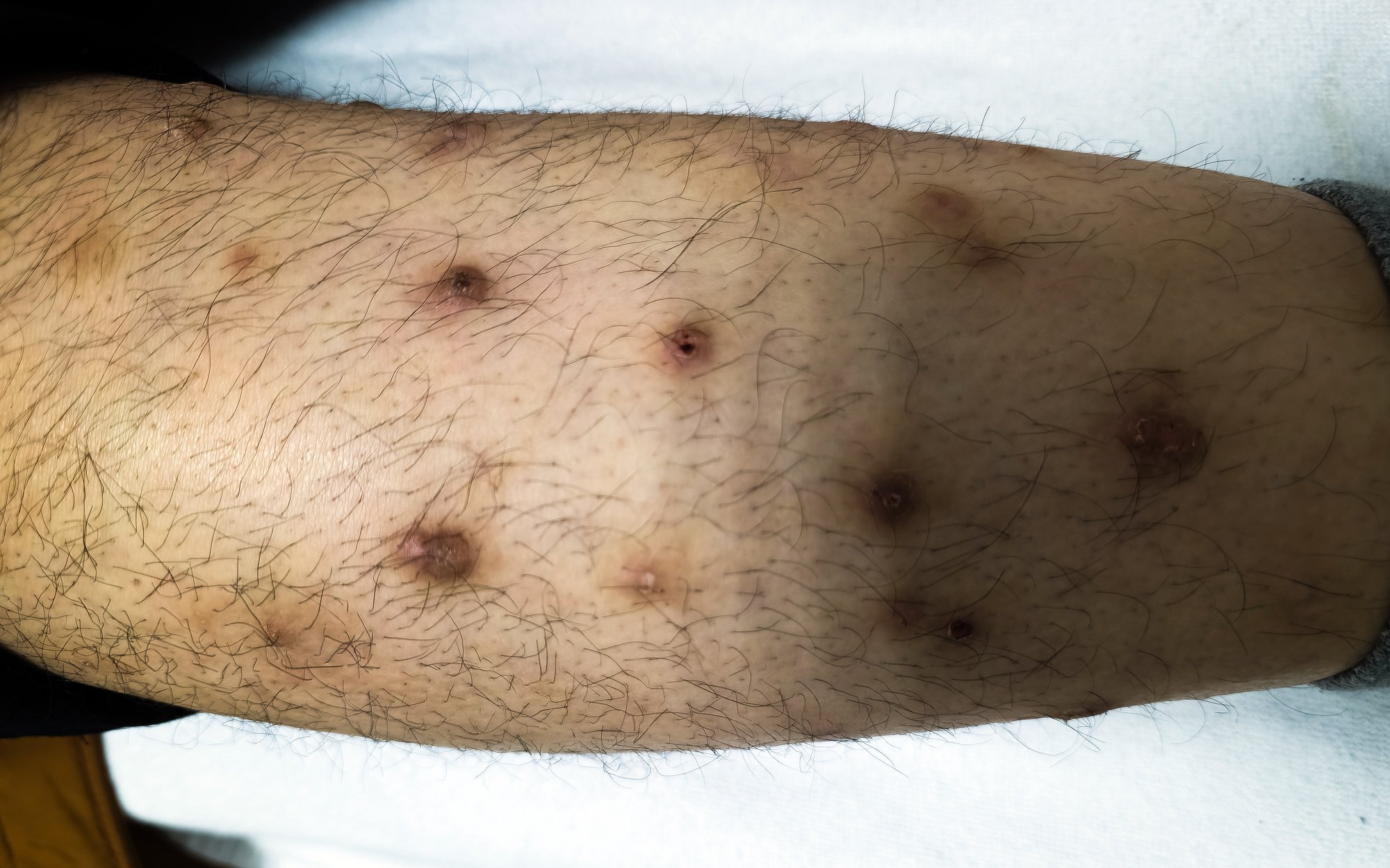
Misdiagnoses are relatively common, the speaker pointed out. Confusion exists, for example, with folliculitis, dermatofibromas, lipomas or angiomas and sometimes Merkel cell carcinoma is misinterpreted as basal cell carcinoma, spinalioma, melanoma or sarcoma. Merkel cell carcinoma (MCC) develops rapidly, usually over weeks to a few months, as an asymptomatic, bulging, reddish-purple tumor with a smooth, usually shiny surface (Fig. 1). On dermoscopy, MCC present as pink, featureless areas, with white fine lines, ulcerations can also be seen in advanced stages, satellite metastases are more common. The preferred site of MCC is the chronically sun-damaged skin of the head and neck region (more than half of cases) and distal extremities (about one-third of cases) [2]. In addition to UV exposure as a significant risk factor of MCC, there is an association with Merkel cell polyomavirus (MCPyV) in over half of patients [2].
In addition to the German-language s2k guideline on Merkel cell carcinoma by Becker et al. an English-language guideline was also published in 2022 [2,3]. The speaker went into more detail about some important guideline recommendations on diagnostics and therapy.
How do you recognize high-risk MCC?
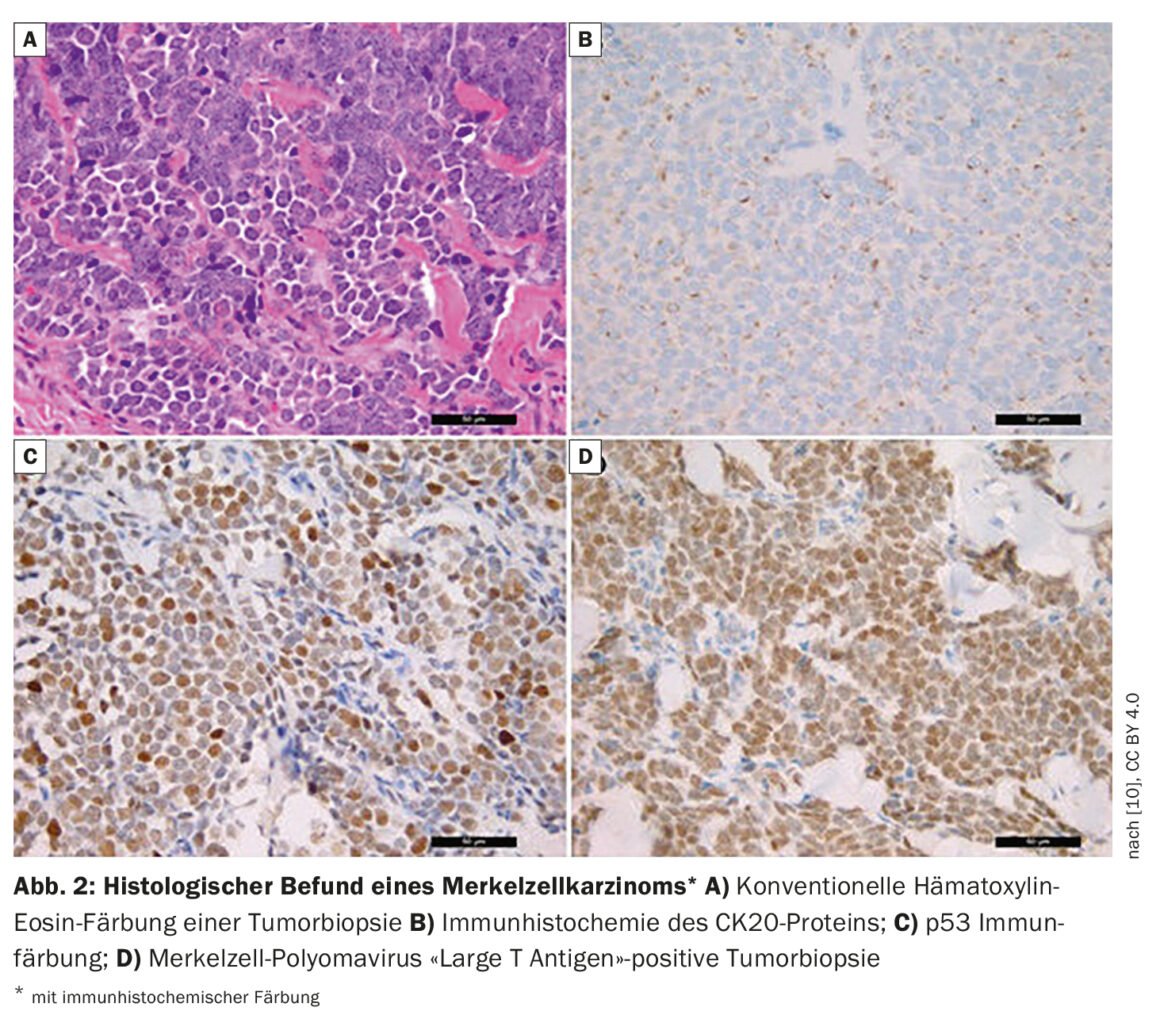
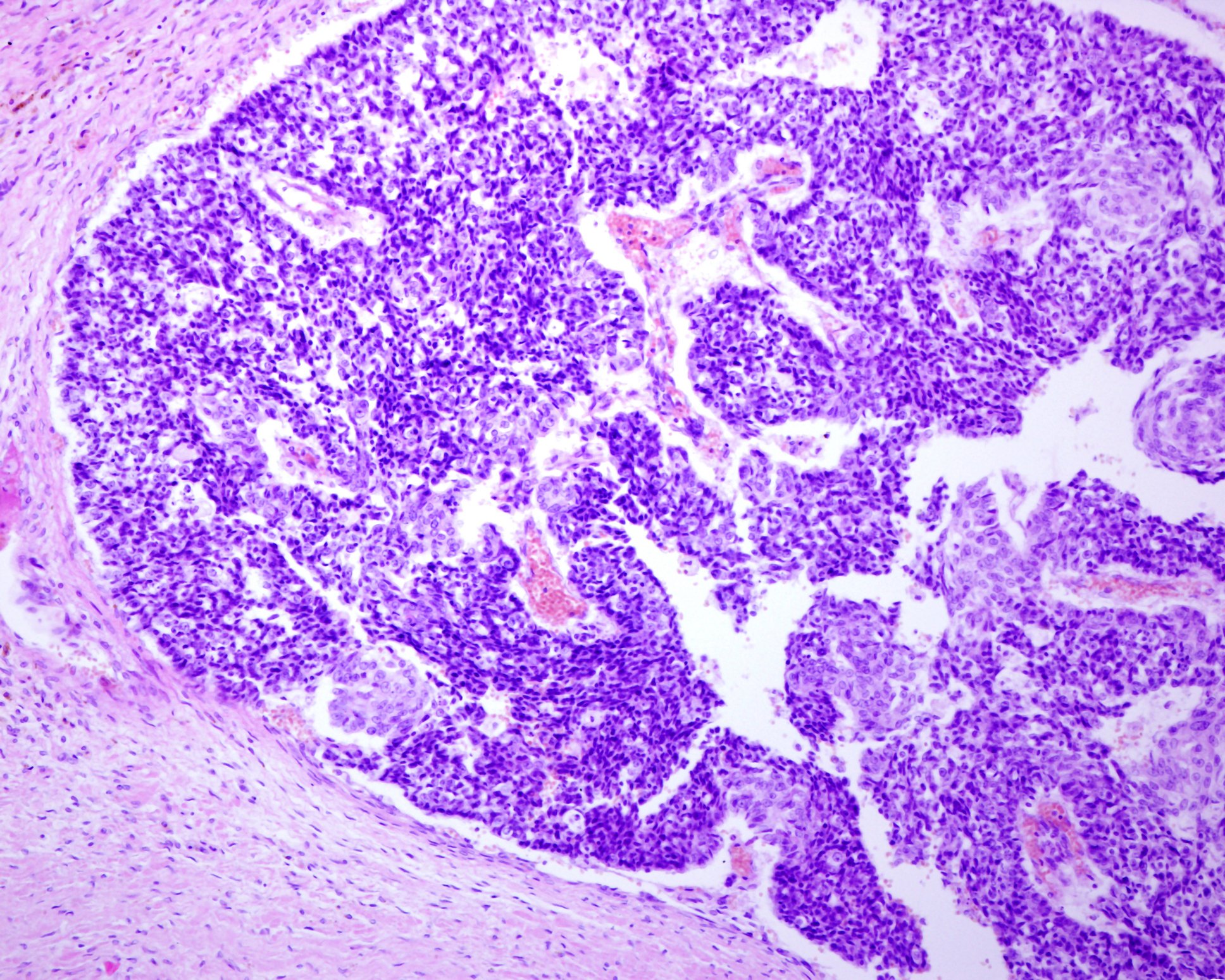
“A quarter of patients already have lymph node metastases at initial diagnosis, and 8% have distant metastases,” said Dr. Wysocki [1]. In patients with suspected MCC, biopsy or total excision is performed in addition to inspection. If MCC has been detected histologically (box, Fig. 2), FDG-PET/CT is performed. If the findings are negative, i.e. the patient is unremarkable with regard to metastases in lymph nodes or other organs, a sentinel lymph node biopsy is performed. Patient characteristics for high-risk MCC include age over 77 years and UV exposure (history of PUVA causes a 100-fold increased risk). Men have a 2.5 times higher risk and also at increased risk are immunosuppressed individuals (HIV, hematologic malignancies, organ transplant recipients). Other criteria for high-risk MCC include tumor size >2 cm, and deep invasion beyond subcutaneous fat. The speaker feels it is important to document tumor thickness and penetration depth in pathology reports. Patients often already have pathologically detectable lymph nodes or there is lymphovascular invasion. The latter would be a high-risk criterion according to NCCN (National Comprehensive Cancer Network)2021 guidelines [4].
Excision: optimal safety distance is difficult to define
The earlier Merkel cell carcinoma is detected and completely removed during surgery, the better the prognosis. “The definition of the safety margin in Merkel cell carcinoma is controversial,” Dr. Wysocki laid out [1]. Some experts suggest taking 1 cm safety distance and subsequent adjuvant re-irradiation. Also tolerable are excision distances <1 cm when followed by adjuvant postirradiation. Mohs surgery is as safe as choosing large safety margins for a standard local excision. This is, among other things, clear from a publication by Carrasquillo et al. published in 2022. forth [5]. If one does not use adjuvant postirradiation, one should maintain safety distances of about 2 cm. “But the optimal safety distance is difficult to define and must be determined on a case-by-case basis in the tumor board,” the speaker emphasized [1].
Experts advise adjuvant radiotherapy
After surgery, adjuvant radiotherapy of the tumor bed and surrounding lymphatic drainage pathways can reduce the risk of disease recurrence. However, adjuvant radiotherapy is not only important to prevent recurrence, but also improves overall survival (OS), disease-free survival, and counteracts the occurrence of distant metastases, Dr. Wysocki stated [1,3]. Adjuvant irradiation of the primary tumor bed should be performed within 8 weeks after surgical excision with a total dose of 50-55 Gy for tumor localization in the head and neck or 60-66 Gy for microscopic and macroscopic positive margins. “When we re-excise the primary tumor, we also do the sentinel node biopsy at the same time if the staging was unremarkable,” the speaker reported [1]. This is because about a quarter of MCC patients already have microscopic lymph node metastases at the time of primary tumor. However, this is decided on an individual basis depending on the general condition of the patient, the anesthetic capability and the localization. At Lucerne Cantonal Hospital, all MCC cases are discussed at the interdisciplinary tumor board. Radiation therapy may also be used to complement surgery and systemic therapy for metastatic Merkel cell tumors. A consensus-based recommendation is that if sentinel lymph node biopsy is positive, adjuvant postradiation should be performed in the lymph node region or combined with completion lymph node dissection.
anti-PDL-1 antibody avelumab – better outcomes than chemotherapy
Previously, chemotherapies had a median OS of only 9 months in the metastatic setting. The anti-PDL-1 antibodies represent a major advance in this regard, Dr. Wysocki emphasized [1]. The anti-PD-L1 antibody avelumab (Bavencio®) has been approved in Switzerland for metastatic MCC (mCC) since 2017 [6]. In the phase II JAVELIN Merkel 2000 trial, avelumab was evaluated in patients who had already received at least one prior cytostatic therapy for MCC [7,8].
In the 88 patients studied with second-line avelumab, a 33% objective response rate was achieved after a median follow-up of 65 months; the median duration of response was 40.5 months [7]. The median progression-free survival (PFS) was 2.7 months and the PFS rate at 24 months was 26%. The median OS was 12.6 months, and the OS rate was 31% at 42 months. These were very good results compared to chemotherapy, the speaker summarized [2]. The study was subsequently extended to include patients in the first line of therapy [9]. Of 116 patients treated with avelumab as first-line (median follow-up: 21.2 months), 35 patients responded over a period of ≥6 months, corresponding to a durable response rate (DRR) of 30.2%. The overall response rate (ORR) was 39.7%. The median PFS was 4.1 months, and the 1-year OS was about 60%, slightly higher than with avelumab as first-line therapy, the speaker explained [1,2]. Therapy-induced adverse events occurred in 81% of avelumab-treated patients but were mostly mild (fatigue, weakness, pruritus). Serious adverse events (mostly grade 3 or 4) were documented in 14.7% of study participants.
Overall, the conclusion regarding Avelumab as a therapeutic option is extremely positive. “We can really offer patients a good systemic therapy,” Dr. Wysocki summarized [1]. The recommended dosage is 10 mg/kg every two weeks. Treatment is given until progression or severe side effects occur. “Unfortunately, there is no approval yet for adjuvant or neoadjuvant treatment,” she said [1].
Congress: Swiss Derma Day
Literature:
- «Update Merkel cell carcinoma», Dr. med. Anja Wysocki, Swiss Derma Day 12.01.2023
- Becker JC, et al.: S2k-Leitlinie – Merkelzellkarzinom – Update 2022. J Dtsch Dermatol Ges 2023; 21(3): 305–317.
- Gauci ML, et al.: European Dermatology Forum (EDF), the European Association of Dermato-Oncology (EADO) and the European Organization for Research and Treatment of Cancer (EORTC). Diagnosis and treatment of Merkel cell carcinoma: European consensus-based interdisciplinary guideline – Update 2022. Eur J Cancer 2022; 171: 203–231.
- NCCN Clinical Practice Guidelines in Oncology: Merkel Cell Carcinoma, Version 1.2021 — February 18, 2021, https://merkelcell.org/wp-content/uploads/2021/02/NCCN-2021.pdf, (last accessed 21.03.2023)
- Carrasquillo OY, et al.: Mohs Micrographic Surgery Versus Wide Local Excision in the Treatment of Merkel Cell Carcinoma: A Systematic Review. Dermatol Surg 2022; 48(2): 176–180.
- Drug Information, www.swissmedicinfo.ch,(last accessed Mar. 21, 2023).
- D’Angelo SP, et al.: Avelumab in patients with previously treated metastatic Merkel cell carcinoma (JAVELIN Merkel 200): updated overall survival data after >5 years of follow-up. ESMO Open. 2021; 6(6): 10029.
- Kaufman HL, et al.: Updated efficacy of avelumab in patients with previously treated metastatic Merkel cell carcinoma after ≥ 1 year of follow-up: JAVELIN Merkel 200, a phase 2 clinical trial. J Immunother Cancer 2018; 6(1): 7.
- D’Angelo SP, et al.: First-line avelumab in a cohort of 116 patients with metastatic Merkel cell carcinoma (JAVELIN Merkel 200): primary and biomarker analyses of a phase II study. J Immunother Cancer 2021; 9(7): e002646.
- Mokánszki A, et al.: Molecular Profiling of Merkel Cell Polyomavirus-Associated Merkel Cell Carcinoma and Cutaneous Melanoma. Diagnostics 2021, 11, 212. https://doi.org/10.3390/diagnostics11020212, www.mdpi.com/2075-4418/11/2/212, (last accessed 21.03.2023)
DERMATOLOGIE PRAXIS 2023; 33(2): 45–46
InFo ONKOLOGIE & HÄMATOLOGIE 2023; 11(2): 22–23