Compared to the past, there are now more and more effective systemic therapies available. This has been helped by a surge in innovation that has produced a wide range of modern anti-inflammatory agents over the last two decades. Patients should be involved in choosing the best possible treatment. Clinical studies and projects in a real-world setting – such as the SDNTT psoriasis registry with a focus on biologic therapies – are constantly providing new and interesting findings.
PD Dr. med. Julia-Tatjana Maul, Senior Physician, Head of Psoriasis and Hidradenitis Suppurativa Consultation and Head of Immunodermatology and Clinical Studies, Department of Dermatology, University Hospital Zurich, gave an up-to-date overview of psoriasis [1]. “Patients with psoriasis are exposed to a great deal of emotional and social stress, which greatly impairs their quality of life,” emphasized the speaker [1]. Not only the symptoms directly associated with the skin manifestations contribute to this, but also frequent comorbidities such as psoriatic arthritis (PsA), cardiovascular diseases, metabolic syndrome and mental disorders.
In terms of aetiopathogenesis, psoriasis is a chronic T-cell-mediated disease in which the Th17/interleukin (IL)-23 axis plays a central role [2,3]. The most common phenotype – psoriasis vulgaris / plaque psoriasis – is characterized by characteristic erythematosquamous plaques in a symmetrical distribution. Predilection sites are mainly the hairy scalp, elbows, knees, umbilical areola, rima ani, (retro)auricular areas [4].
The classification of the severity of plaque psoriasis and the progression measurement is based on BSA (Body Surface Area), PASI (Psoriasis Area and Severity Index) and DLQI (Dermatology Life Quality Index). ** Cases with BSA≤10, PASI≤10 and DLQI≤10 are considered a mild form of psoriasis. If the BSA or PASI values are above 10 and/or the DLQI score is>10, the criteria for moderate to severe psoriasis are met [5]. Various treatment options are available for the treatment of psoriasis, depending on the severity of the disease.
** The higher the DLQI score, which ranges from 0 to 30, the greater the influence on health-related quality of life [31]
The launch of biologics has shaped the last two decades
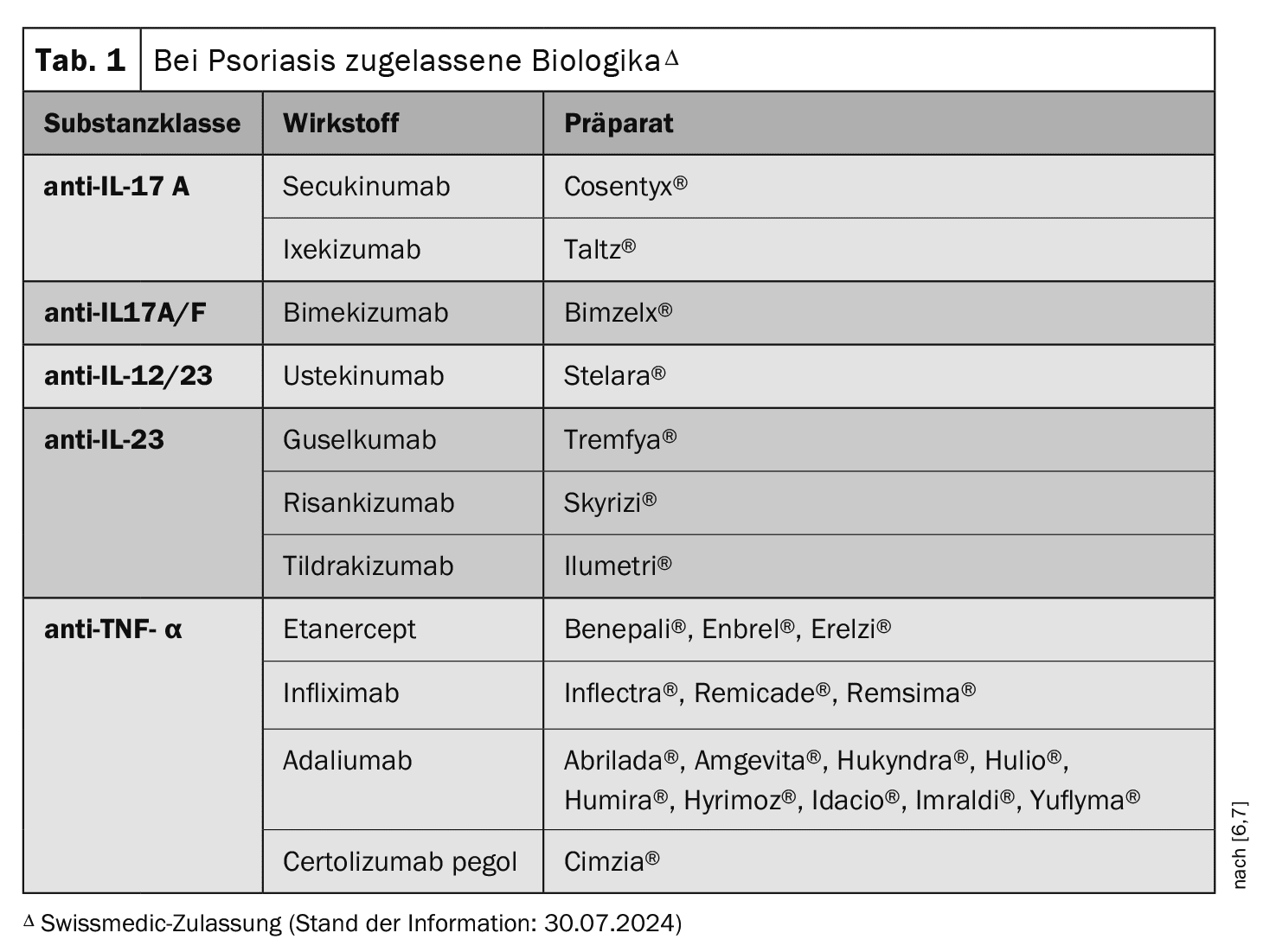
Local therapies (e.g. topical glucocorticoids, vitamin D analogs) are often used for patients with mild to moderate forms of the disease. Phototherapy is carried out using UVA rays (320-400 nm) and UVB rays (280-320 nm) and can be combined with local therapy. Systemic therapy has been revolutionized in recent years by the market approval of biologics (Table 1) [1]. Today, several biologics of different substance classes are available for the treatment of moderate to severe psoriasis in addition to conventional systemic therapeutics (e.g. methotrexate, ciclosporin, acitretin). The limitations applicable in Switzerland stipulate the following requirements for the use of a biologic: at least one therapy trial with at least one conventional systemic therapy and PASI, BSA and/or DLQi >10.
Initially, monoclonal antibodies against TNF-α were approved [6,7]. Etanercept was the first representative of TNF-α inhibitors in the psoriasis indication; infliximab, adalimumab and certolizumab pegol followed later. The first approved interleukin (IL) inhibitor was the anti-IL-12/23-Ak ustekinumab.
This was followed by the anti-IL-17A monoclonal antibodies secukinumab and ixekizumab and the anti-IL-23 antibodies guselkumab, risankizumab and tildrakizumab. Bimekizumab, a dual anti-IL17A/F inhibitor, has now also been approved. The IL-17-A receptor blocker brodalumab is approved in the EU, but not in Switzerland.
An alternative therapeutic approach is phosphodiesterase (PDE)-4 inhibition. Apremilast has been approved in Switzerland for several years and, like the TYK-2 inhibitor deucravacitinib, is a small molecule. Deucravacitinib selectively inhibits tyrosine kinase 2 (TYK2), which belongs to the Janus kinase family, and received EMA approval in 2023. Deucravacitinib& is not currently approved in Switzerland.
& Status of the information: 30.07.2024
Guidelines provide recommendations and real-world studies provide evaluation
The EuroGuiDerm guidelines for the treatment of psoriasis vulgaris published in 2021, in which PD Dr. Maul was one of the contributors, contain evidence-based and consensus-based recommendations for the diagnosis and treatment of psoriasis [8,9]. The German-language S3 guideline “Therapy of psoriasis vulgaris” is essentially based on the EuroGuiDerm guidelines and is periodically updated as a “Living Guideline” [10]: https://register.awmf.org/de/leitlinien/detail/013-001.
Among other things, the criteria-based selection of therapies is discussed in detail. The aim is to create an individual treatment plan for each patient, whereby the presence of comorbidities must also be taken into account.
In registry studies, the efficacy, safety and tolerability of therapies are investigated in a real-world setting. The Swiss Psoriasis Registry “Swiss Dermatology Network for Targeted Therapy” (SDNTT), in which the speaker is involved as Principal Investigator, is a nationwide long-term study that was founded as an initiative of the Swiss Society of Dermatology and Venereology (SGDV) [11]. The focus of this prospective study is on the evaluation of patient benefit and the safety of systemic psoriasis therapies under everyday conditions.
Important findings from the SDNTT project have been summarized in high-ranking scientific articles, including the following publications explained in more detail by PD Dr. Maul in the interview:
- Maul et al. Dermatology 2016: Efficacy and Survival of Systemic Psoriasis Treatments [12]
- Maul et al. BJD 2021: Association of sex and systemic therapy treatment outcomes in psoriasis a two-country, multicentre, prospective, noninterventional registry study [13]
- Maul et al. JEADV 2019: Gender and age significantly determine patient needs and treatment goals in psoriasis – a lesson for practice [14]
- Verardi et al. JEADV 2024: Sex differences in adverse events from systemic treatments for psoriasis: A decade of insights from the Swiss Psoriasis Registry (SDNTT) [15]
- Nielsen et al. Journal of Psoriasis and Psoriatic Arthritis 2024: Predicting Psoriatic Arthritis in Psoriasis Patients – A Swiss Registry Study. Journal of Psoriasis and Psoriatic Arthritis [16].
The international research project Global Healthcare Study on Psoriasis (GHSP; www.dermastudy.org), launched and led by PD Dr. Maul, analyzes access to psoriasis treatment in different geographical regions [1]. While it is taken for granted in Switzerland that patients for whom conventional systemic therapy was not effective will receive access to biologics, this is not always guaranteed in Latin America and other parts of the world, the speaker reported [1]. This is the result of an analysis of a data set with over 1400 patients. The study results were published in the British Journal of Dermatology and served as “Editor’s choice” in April 2024.
Great potential of IL-23-i and IL-17-i – fit is crucial
For patients who qualify for biologic therapy, ideally a biologic should be started immediately after a single conventional therapy and not only after several conventional systemic therapies have been tried. This increases the chance of a good response to therapy, the speaker explained [1]. The biologics directed against IL-23 (guselkumab, tildrakizumab, risankizumab) lead to a blockade of this signaling pathway and have shown a high PASI-90 and PASI-100 response after 52 weeks [17,26,29,30]. Tolerability is generally good and patients with chronic inflammatory bowel disease, multiple sclerosis, tuberculosis or cancer can also be treated with IL-23 inhibitors [8,9,18,19]. The monoclonal antibodies directed against IL-17A (secukinumab, ixekizumab) are also highly effective biologics with a broad evidence base. One advantage of anti-IL-17A antibodies and the IL-17A receptor blocker bimekizumab is that they not only have a favorable effect on plaque psoriasis, but also on all clinical domains of PsA, including radiological progression [20,32].
Using “windows of opportunity” for effective biologics therapy
The speaker emphasized that it is important to provide patients with adequate treatment in good time [1]. There are various study findings that indicate that the response can be improved if biologics are used early in the course of the disease. These include, for example, the GUIDE study on guselkumab and the STEPIn study on secukinumab [21,33].
According to current estimates, over 40% of people with psoriasis are overweight or obese [22]. Overweight psoriasis patients can also benefit greatly from biologics therapy, but it has been shown that their efficacy is somewhat reduced compared to patients of normal weight or that a higher dosage is required to achieve comparable effects. There are several study findings on both IL-23p19 inhibitors [23–26,29,30] and IL-17 inhibitors [27,28] that illustrate this.
Congress: Zurich Dermatology Training Days (ZDFT)
Literature:
- “What’s new – 2023/2024: Psoriasis update”, PD Dr. med. Julia-Tatjana Maul, ZDFT 22-23.06.2024.
- Aggarwal S, et al: Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem 2003; 278(3): 1910-1914.
- Mada S, et al: The Th17/IL-23 Axis and Natural Immunity in Psoriatic Arthritis. Int J Rheumatol 2012; 539683.
- “Safety and efficacy of systemic psoriasis therapy under everyday conditions of a psoriasis specialist consultation”, dissertation, Ioanna Charitidou, 2023, https://refubium.fu-berlin.de, (last accessed 30.07.2024).
- Mrowietz U, et al: Definition of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res 2011; 303(1): 1-10.
- Swissmedic: Medicinal product information, www.swissmedicinfo.ch,(last accessed 30.07.2024).
- Swiss Drug Compendium, https://compendium.ch,(last accessed 30.07.2024).
- Nast A, et al: EuroGuiDerm Guideline on the systemic treatment of Psoriasis vulgaris – Part 1: treatment and monitoring recommendations. JEADV 2020; 34(11): 2461-2498.
- Nast A, et al: EuroGuiDerm Guideline on the systemic treatment of Psoriasis vulgaris – Part 2: specific clinical and comorbid situations. JEADV 2021; 35(2): 281-317.
- Nast A, et al: German S3 guideline on the treatment of psoriasis vulgaris, adapted from EuroGuiDerm – Part 1: Treatment goals and treatment recommendations. JDDG 2021; 19(6): 934-951.
- Swiss Dermatology Network for Targeted Therapies (SDNTT), www.derma.swiss/fachpersonen/forschungsregister/swiss-dermatology-network-for-targeted-therapies-sdntt,(last accessed 30.07.2024).
- Maul JT, et al: Efficacy and Survival of Systemic Psoriasis Treatments. Dermatology 2016; 232(6): 640-647.
- Maul JT, et al: Association of sex and systemic therapy treatment outcomes in psoriasis a two-country, multicentre, prospective, noninterventional registry study. Br J Dermatol 2021; 185(6): 1160-1168.
- Maul JT, et al: Gender and age significantly determine patient needs and treatment goals in psoriasis – a lesson for practice. JEADV 2019; 33(4): 700-708.
- Verardi F, et al: Sex differences in adverse events from systemic treatments for psoriasis: A decade of insights from the Swiss Psoriasis Registry (SDNTT). JEADV 2024; 38(4): 719-731.
- Nielsen ML, et al: Predicting Psoriatic Arthritis in Psoriasis Patients – A Swiss Registry Study. Journal of Psoriasis and Psoriatic Arthritis 2024; 9(2): 41-50.
- Blauvelt A, et al: Efficacy of guselkumab versus secukinumab in subpopulations of patients with moderate-to-severe plaque psoriasis: results from the ECLIPSE study. J Dermatolog Treat 2022; 33(4): 2317-2324.
- “Mastering the Use of IL-23 Inhibitors in Psoriasis and Psoriatic Arthritis”, Joel Gelfand, MD. In: A Report from the 2024 AAD Annual Meeting, Lucas Galimany, MSc/MD.
- Philippot Q, et al: Human IL-23 is Essential for IFN-Gamma-Dependent Immunity to Mycobacteria. Sci Immunol 2023; 8(80): eabq5204.
- “Practical Considerations in Using IL-17 Inhibitors for Psoriasis and Psoriatic Arthritis”, Joseph Merola MD/MSc. In: A Report from the 2024 AAD Annual Meeting, Lucas Galimany, MSc/MD.
- Iversen L, et al: Secukinumab demonstrates superiority over narrow-band ultraviolet B phototherapy in new-onset moderate to severe plaque psoriasis patients: Week 52 results from the STEPIn study. JEADV 2023; 37(5): 1004-1016.
- “Diabetological approach can improve psoriasis”, www.aerzteblatt.de/archiv/236951/Diabetologischer-Ansatz-kann-Psoriasis-bessern,(last accessed 30.07.2024)
- Del Alcázar E, et al: Effectiveness and safety of guselkumab for the treatment of psoriasis in real-world settings at 24 weeks: A retrospective, observational, multicentre study by the Spanish Psoriasis Group. Dermatol Ther 2022; 35(2): e15231.
- Gerdes S, et al: Real-world effectiveness of guselkumab in patients with psoriasis: Health-related quality of life and efficacy data from the noninterventional, prospective, German multicenter PERSIST trial.
J Dermatol 2021; 48(12): 1854-1862. - Hansel K, et al: A multicenter study on effectiveness and safety of risankizumab in psoriasis: an Italian 16-weekreal-life experience during the COVID-19 pandemic. JEADV 2021; 35(3): e169-e170.
- Mastorino L, et al: Risankizumab shows high efficacy and maintenance in improvement of response until week 52. Dermatol Ther 2022 May; 35(5): e15378.
- Herrera-Acosta E, et al: Ixekizumab vs ustekinumab for skin clearance in patients with moderate to severe psoriasis after a year of treatment: Real-world practice. Dermatol Ther 2020 Nov; 33(6): e14202.
- Megna M, et al: Effectiveness and safety of secukinumab in Italian patients with psoriasis: an 84 week, multicenter, retrospective real-world study. Expert Opin Biol Ther 2019; 19(8): 855-861.
- Thaci D, et al: Five-year efficacy and safety of tildrakizumab in patients with moderate-to-severe psoriasis who respond at week 28: pooled analyses of two randomized phase III clinical trials (reSURFACE 1 and re-SURFACE 2). BJD 2021; 185(2): 323-334.
- Reich K, et al: Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomized controlled, phase 3 trials. Lancet 2017; 390: 276-288.
- Finlay AY, Khan GK: Dermatology Life Quality Index (DLQI) – a simple practical measure for routine clinical use. Clin Exp Dermatol 1994; 19(3): 210-216.
- Merola JF, et al: Bimekizumab in patients with active psoriatic arthritis and previous inadequate response or intolerance to tumor necrosis factor-α inhibitors: a randomized, double-blind, placebo-controlled, phase 3 trial (BE COMPLETE). Lancet 2023 Jan 07; 401(10370): 38-48.
- Schäkel K, et al: Early disease intervention with guselkumab in psoriasis leads to a higher rate of stable complete skin clearance (‘clinical super response’): Week 28 results from the ongoing phase IIIb randomized, double-blind, parallel-group, GUIDE study. JEADV 2023; 37(10): 2016-2027.
DERMATOLOGIE PRAXIS 2024; 34(5): 31-32 (published on 28.10.24, ahead of print)