An improved understanding of the pathogenesis of psoriatic diseases has contributed to a new generation of biologics in recent years. The currently approved modern representatives of this group of system therapeutics all interact with the Th17 immune response. The multitude of systemic therapy options has made the choice of the individually appropriate treatment more challenging.
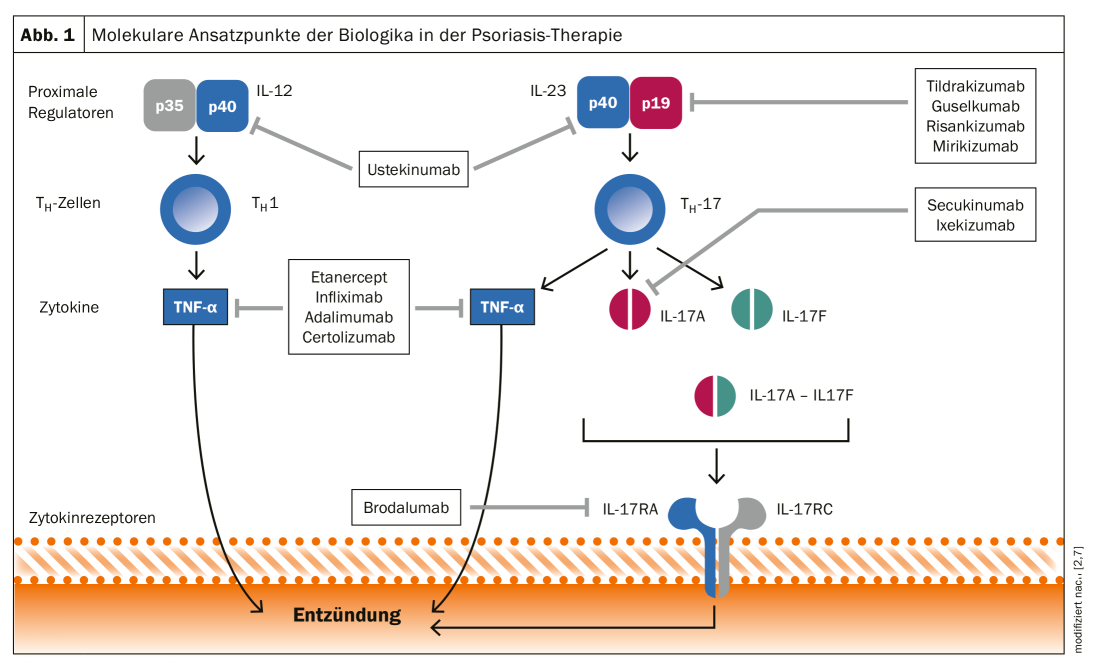
The range of systemically applicable agents has expanded significantly in the last two decades. While the spectrum for the treatment of moderate to severe plaque psoriasis* was initially limited to the oral system therapies methotrexate, ciclosporin, acitretin, dimethyl fumarate and apremilast, a large number of more modern biologics and biosimilars are now available in addition to TNF-α inhibitors. This development is based on an increasingly sophisticated understanding of the pathogenetic basis of psoriasis. As is now known, the Th17 immune response in particular, with its cytokines IL23 and IL17, plays a central role [10]. This is the basis for the new generation of biologics that interact with the Th17 immune response (Fig. 1) [2].
* BSA = Body Surface Area >10%, PASI = Psoriasis Area and Severity Index >10, DLQI = Dermatological Quality of Life Index >10
According to current understanding, psoriasis is considered a systemic disease in which there is a genetically determined dysregulation of the immune system and autoimmunity against the body’s own antigens of the skin occurs. Comorbid disorders are also common. In psoriatic arthritis, rheumatic joint complaints are characteristic in addition to skin manifestations. Furthermore, bowel disease, obesity, diabetes, and cardiovascular disease are commonly reported comorbidities. In addition, the prevalence of mental illnesses such as anxiety disorders and depression is also increased in psoriatics. While PASI-50 or PASI-75 used to be realistic goals for psoriasis treatment, nowadays PASI-90 and PASI-100 are within the realm of possibility [3]. However, with the large number of available biologics, selecting the optimal individual therapeutic agent for the treatment of moderate to severe psoriasis becomes a challenge.
Patient-adapted therapy
The decision factors for system therapy can be divided into four clusters (Overview 1): Clinical criteria, drug characteristics, patient characteristics, approval, and regulation [8]. In addition to the evidence-based recommendations of current guidelines, further criteria should be clarified in individual cases as a basis for the selection of treatment. Further studies are needed to better understand factors such as long-term efficacy, duration to response, safety, and impact on comorbidities. Valid empirical data may facilitate offering an individually appropriate therapy characterized by long-term appearance-free skin, long “drug survival”, and high patient satisfaction scores [9]. It is also important to consider patient preferences. In general, rapid improvement of skin symptoms and freedom from lesions are among the most important criteria for success [1]. According to data from the Swiss Dermatology Network of Targeted Therapies (SDTT), age and gender are stratifying factors with regard to patient needs. While patients over 65 years of age rate a lower frequency of physician consultations, good tolerability of the medication, as well as sleep quality and confidence in the therapy as important, younger patients are more likely to consider it important that their social life is not restricted [1].

Loss of effect as a challenge
One of the greatest challenges in the advancement of systemic psoriasis therapeutics relates to the secondary loss of effect of biologics [3]. After a certain period of treatment, the therapeutic effect decreases with the consequence that the patient switches to another active substance. In this context, one also speaks of “drug survival”, i.e. the duration from the initiation of a therapy until its discontinuation [4,11]. Predictors of “drug survival” may be useful for choosing between different biologics [11]. A meta-analysis published in 2019 identified several predictors (including gender, obesity, and psoriatic arthritis) that were found to be relevant to treatment discontinuation or continuation using data from 16 cohort studies [12].
There is also a psychological component: In psoriasis sufferers who have already undergone several different biologic treatments, the knowledge of a possible renewed loss of efficacy may override patient satisfaction scores. What is hoped for in the future is a systemic treatment whose efficacy lasts for ten years or more. Evidence of durable treatment results from long-term studies brings us closer to this goal.
Molecular targets of different biologics
Psoriasis is an inflammatory disease mediated by T lymphocytes. The immune system is activated and T helper (Th) cells produce various cytokines in the skin, which act on epidermal keratinocytes and lead to the excessive proliferation and abnormal differentiation of keratinocytes characteristic of psoriasis, as well as infiltration with inflammatory cells [2]. Th17 cells, which produce various inflammation-mediating messengers, play a key role [5]. External trigger factors cause interaction between skin-resident keratinocytes, Th17 lymphocytes, and dermal dendritic cells and macrophages [6]. Activation of Th17 lymphocytes induces the release of proinflammatory cytokines such as IL-17A, IL-17F, IL-22, and tumor necrosis factor(TNF)-α. These, in turn, act on keratinocytes and contribute to epidermal hyperplasia, acanthosis, and hyperparakeratosis, as well as endothelial activation, leading to further recruitment of proinflammatory cells such as neutrophil granulocytes into cutaneous tissues [6].
Stimulation by trigger factors, such as stress, infections, or smoking, can trigger the production of IL-6 and IL-23 in genetically predisposed individuals, which promotes the differentiation of IL-17A-producing CD4+ T cells (Th17 cells). On the other hand, Th1 cells are involved, which mainly produce interferon-γ, resulting, among other things, in the destruction of keratinocytes of the basal layer [5].
The different points of attack of various representatives from the group of biologics are shown schematically in Figure 1 [2]. Meanwhile, anti-IL12/23 antibodies (ustekinumab), IL17A inhibitors (secukinumab, ixekizumab) and IL17 receptor blockers (brodalumab) as well as IL23 antagonists (risankizumab, guskumab, tildrakizumab) are available in addition to TNF-α antagonists (adalimumab, certolizumab, etanercept and infliximab) [6]. While the IL12/IL23 inhibitor ustekinumab binds to the p40 subunit of the cytokines IL12 and IL23, the new IL23 inhibitors (risankizumab, guselkumab, tildrakizumab) inhibit the p19 subunit of IL23.
Literature:
- Maul JT, et al: Gender and age significantly determine patient needs and treatment goals in psoriasis – a lesson for practice. J Eur Acad Dermatol Venereol 2019, 33(4): 700-708.
- Sator P: State of the Art: Therapy of Psoriasis vulgaris. Focus: Skin and rheumatism, FdR 01|2019, 04/16/2019
- Yawalkar N: Psoriasis without Symptoms – the New Hope for Patients. SGDV Annual Meeting, Basel 20.09.2019.
- Egeberg A, Bryld LE, Skov L: Drug survival of secukinumab and ixekizumab for moderate-to-severe plaque psoriasis. J Am Acad Dermatol 2019; 81(1): 173-178.
- Eyerich K: Psoriasis – Where are we heading? Key Lecture 3, slide presentation, Prof. Dr. med. Kilian Eyerich, TU Munich, SGDV Annual Meeting, Basel 20.09.2019.
- Wild J, Wegner J, Karbach S: From the skin to the vascular system – psoriasis and cardiovascular risk. Cardiology 2020; 14, 205-211.
- Bartlett HS, Million RP: Targeting the IL-17-T(H)17 pathway. Nat Rev Drug Discov 2015; 14(1): 11-12.
- Augustin M: S09/04 Differentiation factors in therapy decision-making, JDDG 2019, https://onlinelibrary.wiley.com/doi/10.1111/ddg.13793
- Kamata M, Tada Y : Efficacy and Safety of Biologics for Psoriasis and Psoriatic Arthritis and Their Impact on Comorbidities: A Literature Review. Int J Mol Sci 2020; 21(5): 1690.
- Volc S, Ghoreschi K: Pathophysiological basis of systems therapies for psoriasis. JDDG 2016. DOI: 10.1111/ddg.13050, https://onlinelibrary.wiley.com/doi/pdf/10.1111/ddg.13050_g
- Mourad A, et al: Factors predicting persistence of biologic drugs in psoriasis: a systematic review and meta-analysis. Br J Dermatol 2019; 181: 450-458.
- Yiu ZZN: Drug survival of adalimumab, ustekinumab and secukinumab in patients with psoriasis: a prospective cohort study from the British Association of Dermatologists Biologics and Immunomodulators Register (BADBIR). BJD 2020; 183(2): 294-302.
DERMATOLOGY PRACTICE 2020; 30(5): 30-32