At this year’s congress of the Swiss Society of Allergology and Immunology (SGAI), current developments in the fields of therapy and research were highlighted. In addition to new therapeutic approaches for atopic dermatitis, recent study results on drug treatment of mastocytosis (omalizumab) and hereditary angioedema (lanadelumab) were presented.
This year, the Swiss Society of Allergology and Immunology (SGAI) held its annual congress jointly with the Swiss Society of Rheumatology (SGR). As the President of the SGAI, Prof. Dr. med. Daniel Speiser, University of Lausanne, and the President of the SGR, Prof. Dr. med. Diego Kyburz, University Hospital Basel, emphasized in their welcoming remarks, the two specialties share many common interests, since immunology also occupies a central position in the care of patients with rheumatological diseases.
The year in review
In one of the sessions, a review of the year focused on allergy-related topics was presented. Prof. Dr. med. Schmid-Grendelmeier, Senior Physician Allergology at the University Hospital Zurich, was responsible for the field of allergic skin diseases and focused here on atopic dermatitis (AD). “After a long time of nothing happening in this area, I can now report some news after all,” he explained. Thus, it would have turned out by now that an AD is much more heterogeneous than previously assumed. For example, using skin biopsies, it has been shown that there are immunological differences between AD in infants and adults [1]. “This could also have an impact on the success of a treatment,” Prof. Schmid-Grendelmeier said.
In the context of new therapeutic approaches, the speaker pointed out the good study results now available on dupilumab, a monoclonal antibody binding to the α-subunit of the interleukin-4 and interleukin-13 receptor, in patients with moderate-to-severe AD [2]. In a one-year randomized, placebo-controlled phase III study, dupilumab (in combination with topical steroids) was shown to have a sustained effect in AD patients over this longer period [3]. “But if we stop treatment, the disease will come back,” Prof. Schmid-Grendelmeier emphasized. From his own experience, he reported, “We’ve been able to use dupilumab so far in several patients with severe, refractory disease and achieved very great improvement in some of them.” On the subject of side effects, he pointed in particular to conjunctivitis. “This does not occur that infrequently and may well necessitate the use of topical steroids.”
Another compound currently also in clinical trials in patients with AD is the anti-interleukin-31 receptor antibody nemolizumab [4]. “This antibody showed a good effect, but almost exclusively on itching,” Prof. Schmid-Grendelmeier pointed out.
Placebo-controlled study of omalizumab in mastocytosis.
Distler et al. used the anti-IgE antibody omalizumab in their double-blind, placebo-controlled multicenter study in seven patients (66% women) with various forms of mastocytosis, and nine patients (85.7% women) received placebo [5]. Omalizumab was dosed according to body weight and total serum IgE, as is standard practice for its use in asthma patients. The results of the work were presented as a poster at the congress. After six months of treatment, the median AFIRMM (Association Française pour les Initiatives de Recherche sur le Mastocyte et les Mastocytoses) score had improved from 104.0 to 102.0 with placebo and from 52.0 to 26.0 with omalizumab (p=0.286). The extent of improvement did not differ significantly (p=0.941). The authors then stated that interpretation of the results of this, to their knowledge, first double-blind, placebo-controlled study of omalizumab in mastocytosis was difficult because of the small number of patients and the differences in baseline characteristics of the two groups. However, in addition to improvement in AFIRRM score, they also observed improvement in several mastocytosis-associated symptoms, including, in particular, diarrhea, dizziness, flushing, and anaphylactic reactions. Side effects of treatment were rare and comparably frequent in both groups. Larger studies will be needed to confirm these results.
Lanadelumab in hereditary angioedema.
Lanadelumab is a fully human, potent, specific, and long-lasting anti-Kallikrein antibody [6]. In patients with hereditary angioedema (HAE) type I/II, lanadelumab (150 mg every four weeks, 300 mg every four weeks, 300 mg every two weeks versus placebo) was shown to be effective and safe in preventing attacks over a 26-week period (days 0 to 182) in the phase III HELP trial [7]. A paper presented in Interlaken now examined the efficacy profile of the antibody during the steady-state phase of the study (days 70 to 182, 16 weeks of treatment) [8].
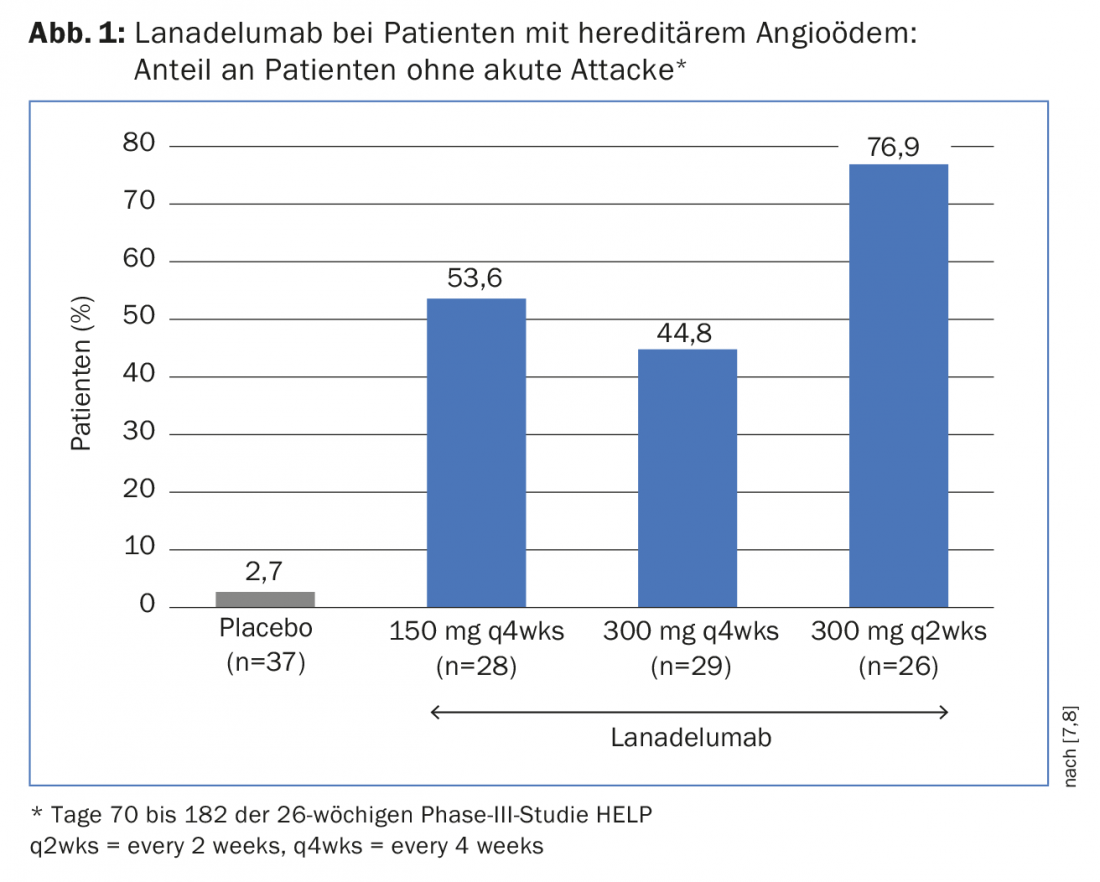
Of the patients treated with 300 mg lanadelumab every two weeks, 76.9% remained attack-free during this period, compared with 2.7% of patients in the placebo group (Fig. 1). The mean monthly attack rate was reduced by 91.5% compared with placebo-treated patients. Of 26 patients treated with lanadelumab, one suffered a severe attack, whereas this was the case in 31 of a total of 37 patients in the placebo group. The other two lanadelumab doses also effectively reduced the number of attacks compared with placebo. This analysis thus supports the results of the primary analysis of HELP and again demonstrates the efficacy of lanadelumab as a long-term prophylactic agent for patients with HAE.
Source: SGAI Annual Congress, August 30-31, 2018, Interlaken
Literature:
- Brunner PM, et al: The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J Allergy Clin Immunol 2017; 139(4S): S65-S76.
- Simpson EL, et al: Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med 2016; 375: 2335-2348.
- Blauvelt A, et al: Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet 2017; 389: 2287-2303.
- Ruzicka T, et al: Anti-Interleukin-31 Receptor A Antibody for Atopic Dermatitis. N Engl J Med 2017; 376: 826-835.
- Distler M, et al: The effect of omalizumab in mastocytosis patients. Prospective double-blind, placebo-controlled multicentre study. Swiss Medical Weekly 2018; 148 (Suppl 231): Abstract SSAIO 10.
- Kenniston JA, et al: Inhibition of plasma kallikrein by a highly specific active site blocking antibody. J Biol Chem 2014; 289: 23596-23608.
- Banerji A, et al: Lanadelumab for prevention of attacks in hereditary angioedema: results from the phase 3 HELP study. Ann Allergy Asthma Immunol 2017; 119 (suppl): S5 (Abstract OR034).
- Maurer M, et al: Lanadelumab Is Highly Efficacious at Steady State in Hereditary Angioedema (HAE): Results of the Phase 3 HELP Study. Swiss Medical Weekly 2018; 148 (Suppl 231): Abstract SSAIPeF16.
DERMATOLOGIE PRAXIS 2018; 28(5): 36-37











