A child with unexplained cytopenia and additional symptoms such as fever, lymphadenopathy, or hepatosplenomegaly should be generously and rapidly referred to a center. Hyperleukocytosis, tumor lysis syndrome, and mediastinal tumor are life-threatening complications in the initial evaluation and treatment phase. Survival rates are over 80% for acute lymphoblastic leukemia (ALL) and 60% for acute myeloid leukemia (AML). The early response to therapy and the cytogenetics of the blasts are prognostically decisive – in ALL additionally the age, the leukocyte count and the immunophenotype. Long-term survivors after childhood leukemia are at significantly increased risk for neuropsychological, endocrinological, metabolic, and cardiac late effects, as well as second tumors.
Acute childhood leukemia is an impressive example of the success story of pediatric hematology and oncology over the past 40 years. For example, acute childhood leukemia has evolved from an almost always lethal disease in the 1960s to one that is now frequently curable, with long-term survival of over 80%. The history of leukemia treatment demonstrates the importance of randomized clinical trials, which provide a basis for continuous improvement in leukemia treatment. In the future, new insights into the biology of leukemia cells will allow more targeted, risk-adapted therapy [1].
Epidemiology
In Switzerland, between 50-60 children (0 to 14 years of age) develop acute leukemia each year (incidence 5/100 000 inhabitants) [2]. Acute leukemia is the most common childhood malignancy, accounting for 30% of cases, and is divided 80% between acute lymphoblastic leukemia (ALL) with B- and T-cell ALL, and 20% between acute myeloid leukemia (AML). Patients with ALL have a long-term survival rate of 80% and patients with AML 55-60%. Boys are affected slightly more often than girls. The age distribution shows a peak in frequency between the second and fifth years of life for ALL and in the first and second years of life and from adolescence for AML [1].
Clinical presentation
In acute leukemia, clonal expansion of immature hematopoietic progenitor cells in the bone marrow results in displacement of normal hematopoiesis. This bone marrow insufficiency results in anemia, neutropenia, and thrombocytopenia with symptoms listed below (Table 1). In addition, leukemic infiltration of the bone marrow may result in life-threatening hyperleukocytosis and coagulopathy. In addition, various organs such as liver, spleen, lymph nodes, thymus, testis, skin/mucosa, and CNS can be infiltrated (Table 1) [3]. Most often, the symptoms persist for a few days to weeks.
Diagnostics and classification
If acute leukemia is clinically suspected (see symptoms Tab. 1) a blood count should be performed by a pediatrician or general practitioner. If the blood count shows monocytopenia, bicytopenia or tricytopenia and/or leukocytosis, the child should be generously and quickly referred to a specialized pediatric hospital if possible.

Here, after initial diagnostics for early detection of tumor lysis syndrome or mediastinal mass (differential blood count with microscopic differentiation, liver values, kidney values, electrolytes, uric acid, LDH, coagulation, and chest X-ray), the following leukemia-specific examinations in the bone marrow (bone marrow aspiration and biopsy) are initiated.
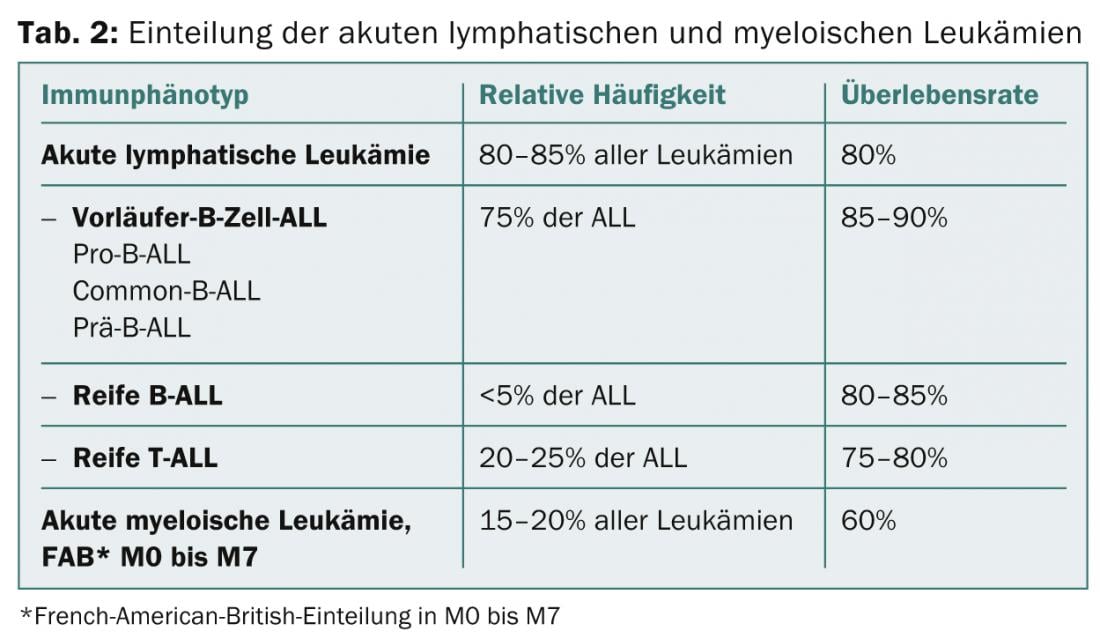
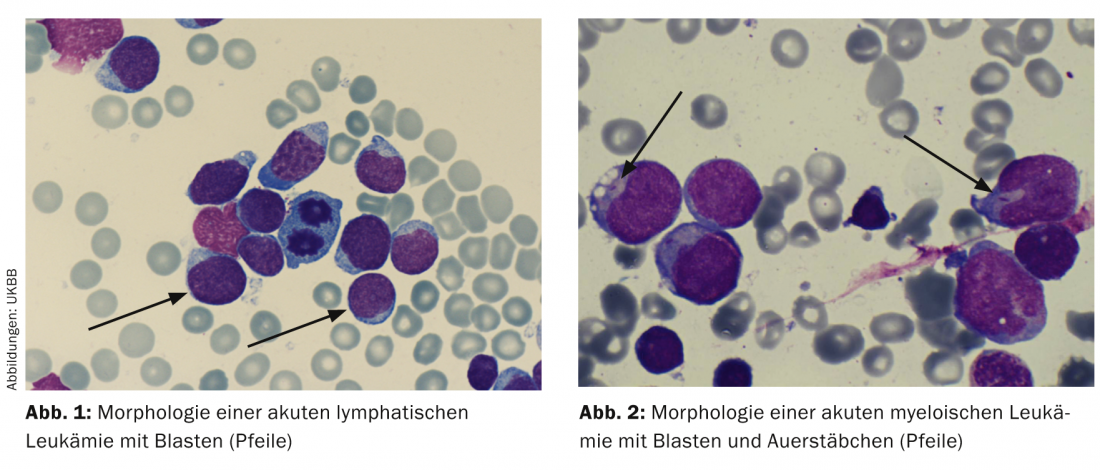
Cytology/morphology: Here, the bone marrow is assessed by light microscopy with regard to its morphology. By definition, ALL has ≥ 25 and AML has ≥ 30% blasts in the bone marrow. Additional cytochemical staining (myeloperoxidase, acid phosphatase, etc) can provide initial clues to leukemia types (Table 2; Figs. 1 and 2) .
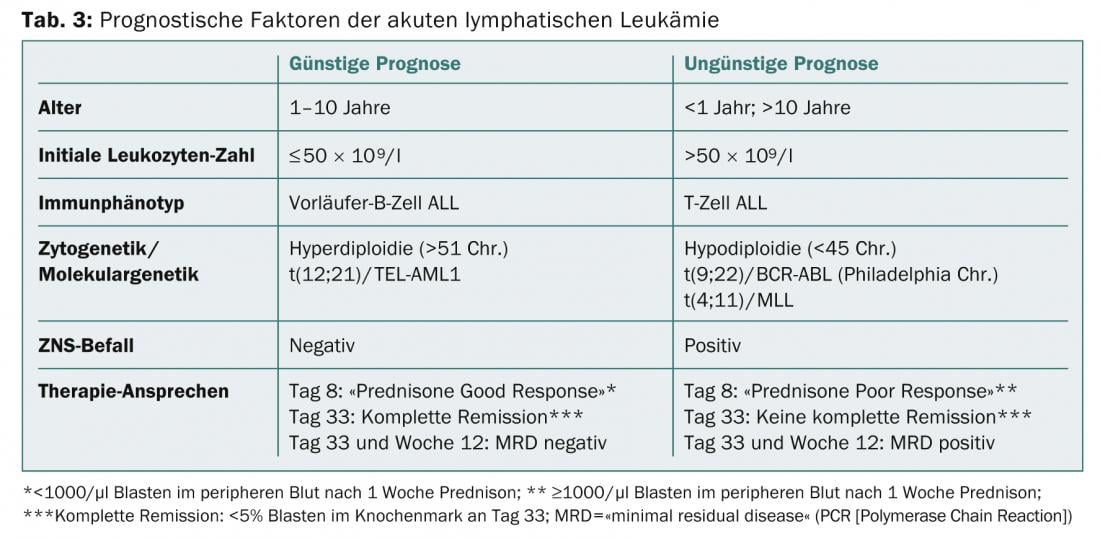
Auer rods prove AML and are an expression of the maturation disorder of the leukemia cell (Fig. 2).
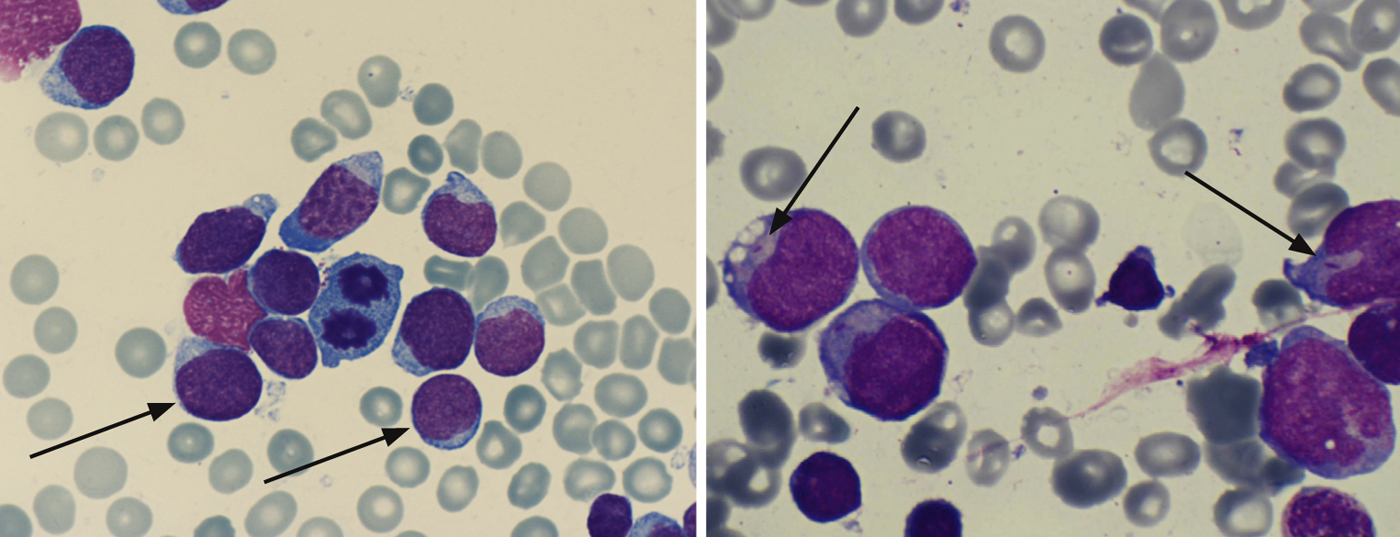
Immunophenotyping: Based on specific surface markers of the leukemic blasts, flow cytometric immunophenotyping is used to characterize the leukemic cells with regard to their maturity and affiliation to the B- or T-cell series or myeloid series (Table 2).
Cytogenetics and molecular genetics: In addition, the acute leukemias can be characterized on the basis of chromosomal aberrations of the blasts and classified into different risk groups. Prognostically favorable in B-ALL are, for example, hyperdiploidy and translocation t(12;21)(p13;q21) with the transfusion gene TEL-AML1; prognostically unfavorable are hypodiploidy and translocation t(9;22)(q34;q11) (= Philadelphia chromosome) with the transfusion gene BCR-ABL ( Table 3) .
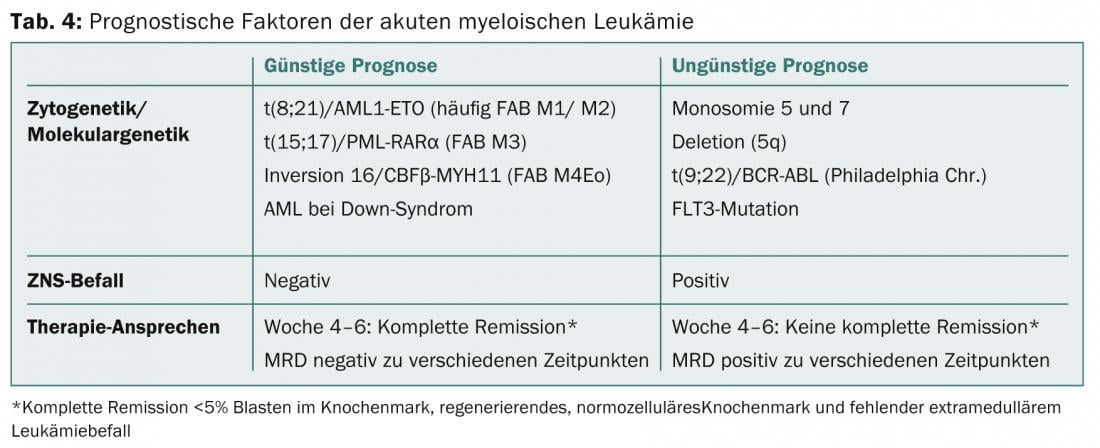
In the diagnosis of AML, detection of translocations t(8;21) and t(15;17) or evidence of inversion 16 implies a more favorable prognosis, whereas detection of monosomy 5 or 7 is associated with a poor prognosis (Table 4) [1,4,5].
Therapy
As mentioned above, the prognosis of childhood acute leukemia has improved impressively during the last 40 years. First, the central nervous system was identified as a frequent site of origin of recurrence and prophylactic CNS treatment was initiated. Second, risk-adapted complex chemotherapy protocols have been developed. In addition, supportive therapy has improved significantly, resulting in a decrease in therapy-associated morbidity and mortality [1].
Nowadays, the therapy of childhood ALL consists of polychemotherapy, which includes steroids and various other chemotherapeutic agents (methotrexate, vincristine, asparaginase, cytarabine, cyclophosphamide, anthracyclines, 6-mercaptopurine). A child with newly diagnosed ALL begins with induction chemotherapy, in which a combination of five to six chemotherapeutic agents is administered for five weeks with the goal of minimizing the leukemia cell burden to a level that is no longer clinically or hematologically detectable. Induction is followed by consolidation chemotherapy to treat the extramedullary compartment (CNS and testis). It consists of intrathecal and high-dose systemic methotrexate administration, which is intended to prevent CNS recurrences and replaces the preventive CNS irradiation that was routinely performed in the past. Reinduction chemotherapy then completes the intensive initial treatment phase after approximately six months. A large part of the intensive chemotherapy phases can be performed on an outpatient basis. Subsequently, outpatient maintenance chemotherapy with 6-mercaptopurine and methotrexate is given for another 18 months.
Hematopoietic stem cell transplantation (HSCT) is used in pediatric patients with a high-risk profile (poor response to therapy, prognostically unfavorable molecular genetics, relapse) and a suitable donor. CNS irradiation is indicated when leukemia cells are initially detected in the CSF [5].
The most important prognostic parameter in childhood leukemia treatment is the response to therapy. A reduction of blasts in the peripheral blood to <1000/µl after one week of steroid treatment (day 8), a complete hematologic remission with <5% blasts in bone marrow smear at the end of induction (day 33) and a negative value of MRD (“minimal residual disease” by PCR) at day 33 and week 12 are the strongest predictors of relapse-free survival (Table 3) [5].
Treatment of childhood AML requires very intensive polychemotherapy consisting of short chemotherapy blocks with anthracyclines, cytarabine, etoposide, 6-thioguanine, and possibly ATRA (“all-trans-retinoid acid”) [3]. Chemotherapy begins with induction, followed by consolidation and intensification, and ends with approximately one year of maintenance chemotherapy with 6-thioguanine and cytarabine. In addition, extramedullary therapy with intrathecal methotrexate, cytarabine, and prednisone is given at regular intervals [6].
According to the current status of international studies, preventive cranial irradiation is not used nowadays [6,7]. Patients with unfavorable cytogenetics, poor response to therapy, or recurrence qualify for HSCT [6].
As in ALL, response to therapy is a critical prognostic factor in AML. Remission in AML is defined with<5% blasts in the bone marrow, a regenerating normocellular bone marrow, and an absence of evidence of extramedullary leukemia. This is usually achieved after four to six weeks of induction therapy [4].
Complications
An important and common therapy-associated side effect of leukemia treatment is chemotherapy-induced myelotoxicity with pancytopenia. It leads via neutropenia to an increased tendency to infection with fever in neutropenia, bacterial, fungal and viral infections, often requiring hospital admission. In addition, there is an increased risk of opportunistic infections (Pneumocystis jirovecii), which is why antibiotic prophylaxis with trimethoprim-sulfamethoxazole is given. In addition, anemia and thrombocytopenia occur, so regular transfusions may be necessary. In addition to bone marrow, other cell series with a high division rate are also damaged. Consequently, there is enoral and gastrointestinal mucositis due to mucosal damage and alopecia due to hair follicle damage. Nausea and vomiting are also common side effects that can usually be treated well with antiemetics. In addition, there are many, but rarer, drug-specific side effects.
Late effects
As the survival rate of children who develop acute leukemia increases, so do the numbers of former pediatric cancer patients with late effects from the disease and treatment [1]. These include neurologic, endocrinologic, metabolic, and cardiac problems, as well as second tumors.
Neurological late effects include neuropsychological deficits, which are primarily a consequence of cranial irradiation. It has been shown that replacement of prophylactic cranial irradiation with intrathecal chemotherapy resulted in a reduction of neuropsychological late effects [8]. Neuropsychological problems include difficulties in arithmetic, verbal short-term memory, executive functions, attention, and concentration.
Endocrinologic and metabolic late effects primarily affect children with cranial irradiation and include growth hormone deficiency, thyroid dysfunction, pubertal delay or pubertas praecox, and metabolic syndrome. Fertility disorders are observed almost exclusively in transplanted patients.
Because of relatively high cumulative anthracycline doses, especially in AML treatment, dilated cardiomyopathy may occur after years or decades as a late cardiac sequela.
Cranial irradiation increases the risk of developing benign and malignant secondary brain tumors (meningiomas, gliomas) and thyroid adenomas or carcinomas. Patients after chemotherapy alone are at increased risk for myelodysplastic syndromes or secondary AML as a manifestation of chemotherapy-induced premalignant or malignant degeneration of the bone marrow [1].
Christina Schindera, MD
Literature:
- Orkin S, et al: Acute Lymphoblastic Leukemia and Myeloid Leukemia, Myelodysplasia, and Myeloproliferative Disease in Children. Oncology of Infancy and Childhood 2009; 297-402. Saunders Elevier.
- Kuehni C: Swiss Childhood Cancer Registry, Annual Report 2011/2012. 2013 www.kinderkrebsregister.ch/fileadmin/KKR08/uploads/pdf/AnnualReport_SCCR_2011_2012_FINAL.pdf.
- Bailey S, Skinner R: Oxford Specialist Handbooks in Paediatrics: Paediatric Haematology and Oncology. 2010. Oxford Medical Publications, Oxford, UK.
- Creutzig U, Reinhardt D: Multicenter Therapy Optimization Study AML-BFM 2004. 2010.
- Schrappe M, AIEOP-BFM ALL 2009: International collaborative treatment protocol for children and adolescents with acute lymphoblastic leukemia. 2013.
- Creutzig U, Dworzak M, Reinhardt D: Acute myeloid leukemia in childhood. 2013. %C3%.
- Pui CH, Howard SC: Current management and challenges of malignant disease in the CNS in paediatric leukaemia. Lancet Oncol 2008; 9(3): 257-268.
- Von der Weid N, et al: Intellectual outcome in children and adolescents with acute lymphoblastic leukaemia treated with chemotherapy alone: age- and sex-related differences. Eur J Cancer 2003; 39(3): 359-365.
InFo Oncology & Hematology 2014; 2(5): 4-7.