Two studies presented at the 2015 Gastrointestinal Cancers Symposium in San Francisco were dedicated to first- and second-line treatment of metastatic colorectal cancer. One was to determine whether the more intensive chemotherapy regimen FOLFOXIRI was superior to the standard regimen FOLFIRI, and the other was to test the addition of ramucirumab to FOLFIRI after failure of initial therapy.
TRIBE is an Italian phase III trial. It compared two first-line chemotherapies in 508 patients with metastatic colorectal cancer: first, the standard treatment FOLFIRI (folinic acid [Leucovorin], 5-fluorouracil, irinotecan [Campto®]) plus bevacizumab, and second, the newer combination FOLFOXIRI (FOLFIRI, oxaliplatin) plus bevacizumab. The results are encouraging. Both therapies were administered for a maximum of twelve cycles (six months), followed by a less intensive maintenance phase with bevacizumab and 5-FU (until progression).
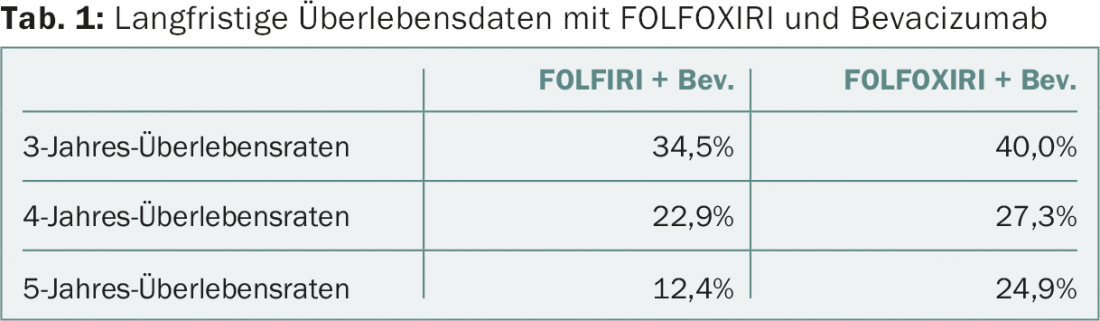
Patients were followed for a median of 48.1 months. Mortality risk was significantly reduced by 20% in the FOLFOXIRI arm: Median overall survival was 29.8 vs. 25.8 months in the comparison (HR 0.80, 95% CI 0.65-0.98, p=0.030). Long-term survival data are shown in Table 1 . Thus, after five years, there was an impressive doubling of survival under the newer combination (although the results should be considered preliminary). Approximately equal numbers of patients from both groups underwent radical resection after induction treatment reduced tumor burden (15% FOLFOXIRI, 12% FOLFIRI). The results regarding the primary endpoint had already been published in October 2014 [1]: The median time of progression-free survival was 12.1 months in the study group and 9.7 months in the other arm (HR 0.75; 95% CI 0.62-0.90; p=0.003).
Who benefits?
For patients who tolerate three chemotherapeutic agents and are otherwise in good health, these findings are hopeful, especially because they confirm the results of an earlier smaller phase III trial (GONO) [2]. There, FOLFOXIRI and FOLFIRI without the addition of bevacizumab had been compared in first-line therapy for metastatic colorectal cancer.
The side effect profile of FOLFOXIRI plus bevacizumab can also be considered acceptable: Although the risk of diarrhea and lower leukocyte levels was higher, serious adverse events did not occur more frequently. Overall, toxicities are controllable. Nevertheless, caution is advised when prescribing this nevertheless very intensive chemotherapy. In particular, patients older than 75 years or elderly (70-75 years) in poor general condition are not eligible for treatment.
In Italy, the follow-up study TRIBE-2 is currently being prepared. Two treatment strategies are compared in 654 patients:
- FOLFOXIRI plus bevacizumab in the first-line setting, followed by reinduction with the same combination after progression.
- FOLFOX plus bevacizumab in the first line, followed by FOLFIRI plus bevacizumab after progression.
Other studies are looking at the possibility of shortening chemotherapy from six to four months and optimizing the maintenance phase (in MACBETH, FOLFOXIRI is combined with cetuximab).
What’s new in the second line?
Promising results for second-line therapy were also presented at the symposium. When initial therapy is no longer effective, metastatic colorectal cancer is difficult to treat. Therefore, progress in the second line, while relatively small at first glance, is significant.
A study presented at the symposium tested the angiogenesis inhibitor ramucirumab in 1072 patients with advanced colorectal cancer that was progressive on or after initial therapy. The addition of ramucirumab, which also targets lung and especially gastric cancer, to the standard therapy FOLFIRI resulted in a survival benefit compared to FOLFIRI alone (plus placebo). Specifically, the RAISE trial showed a significant 16% risk reduction with the addition of 8 mg/kg bw ramucirumab (HR 0.84; 95% CI 0.73-0.98; p=0.0219). Median life was prolonged by approximately one and a half months (13.3 vs. 11.7 months). The researchers also found a significant benefit in progression-free survival: The median difference was 1.2 months (5.7 vs. 4.5; HR 0.79; 95% CI 0.70-0.90; p=0.0005). Results were consistent across subgroups. Both therapies were approximately equally successful in reducing tumor volume (13.4% in the ramucirumab vs. 12.5% in the placebo group).
Expansion of the indications for ramucirumab possible
Adverse events of grade 3 or higher that occurred more frequently in ramucirumab patients were hypertension (11.2 vs. 2.8%), neutropenia (38.4 vs. 23.3%), diarrhea (10.8 vs. 9.7%), and fatigue (11.5 vs. 7.8%). No new or surprising side effects occurred.
The proven activity of ramucirumab against metastatic colorectal cancer argues for an appropriate expansion of second-line therapy – particularly in patients who did not respond to a first-line combination with bevacizumab (RAISE patients had received bevacizumab, oxaliplatin, and a fluoropyrimidine in the first-line setting). The tested compound has an acceptable safety profile and could therefore be a useful addition to standard chemotherapy in the future. So far, bevacizumab and aflibercept as adjuncts have been the most effective in second-line therapy. Because patients with fast-growing tumors were also included in RAISE, the collective can be considered typical of everyday practice.
Further studies testing ramucirumab also in first-line colorectal cancer or in other settings are now indicated. For example, the potential of ramucirumab following first-line therapy that includes cetuximab or as an adjunct to other chemotherapy regimens remains open.
Source: 2015 Gastrointestinal Cancers Symposium (ASCO GI), January 15-17, 2015, San Francisco.
Literature:
- Loupakis F, et al: Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med 2014 Oct 23; 371(17): 1609-1618.
- Falcone A, et al: Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol 2007 May 1; 25(13): 1670-1676.
InFo ONCOLOGY & HEMATOLOGY 2015; 3(3-4): 28-29.











