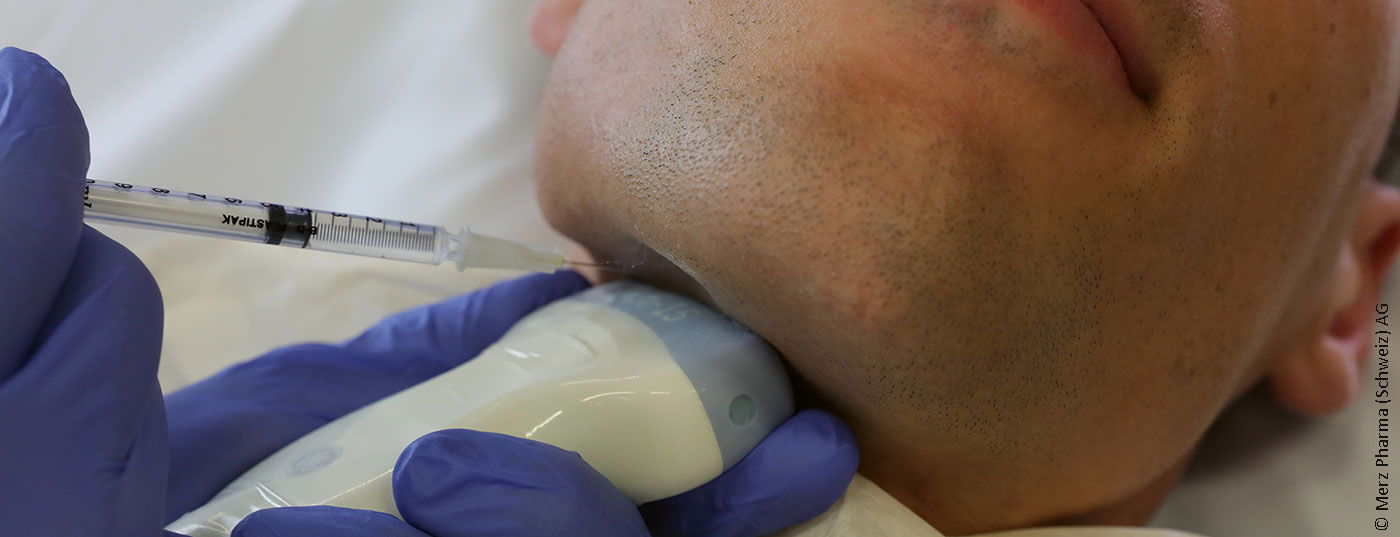
In numerous neurological conditions such as Parkinson’s syndromes, stroke or traumatic brain injury, impaired sialorrhea may occur. IncobotulinumtoxinA is the first drug to be approved in Switzerland for the treatment of chronic sialorrhea in adults. The decisive factor was the SIAXI study – A Commentary.
In many neurological diseases or defective conditions, sialorrhea, i.e. the uncontrolled outflow of saliva from the oral cavity to the outside, can occur. The “SIAXI” study, a multicenter, randomized, double-blind, placebo-controlled Phase III trial, evaluated the efficacy and safety of incobotulinumtoxinA for the treatment of chronic sialorrhea due to Parkinson’s syndromes, stroke, or traumatic brain injury.
Parkinson’s disease patients are particularly affected by sialorrhea at 32-74% and accounted for a majority of the study population (approximately 80% of patients; idiopathic and atypical parkinsonian syndromes). Pathophysiologically, there is no actual overproduction of saliva in this group of patients, but the ability to retain saliva in the oral cavity and swallow it continuously is increasingly limited. Among other factors, impaired head control (antecollis), hypomimia with an open mouth, and decreased tongue and lip mobility play a role. Patients with sialorrhea very often also have dysphagia. As a result, the quality of life is impaired, not only for patients but also for family caregivers. However, skin maceration and pneumonia may also occur, linking sialorrhea to increased mortality. Sialorrhea is shameful and rarely reported spontaneously, so as with many other non-motor symptoms, specific inquiries must be made. Saliva stains on clothing and frequent use of tissues may provide initial clues. Therapy for chronic sialorrhea is multidisciplinary and includes speech therapy and, to date, off-label use of anticholinergic agents, but this is often limited by systemic side effects. In 1997, the use of botulinum toxin was first proposed for the treatment of sialorrhea and, starting in 1999, several smaller prospective studies followed, providing a basis for the off-label use of this group of drugs [1].
Study design
In several centers in Germany and Poland, a total of 184 patients were randomized to receive either incobotulinumtoxinA 100 units, incobotulinumtoxinA 75 units, or placebo (2:2:1). The distribution of the doses to the eq. parotid gland and the submandibular gland was performed in a 3:2 ratio. The injections were performed bilaterally and the practitioners could decide whether to perform the injections with or without ultrasound guidance. The co-primary endpoints were a change in unstimulated salivary flow rate (uSFR) and the Global Impression of Change Scale (GICS), both between the time points immediately before and four weeks after injection. The GICS is a 7-point Likert scale on which patients were asked to indicate how their function had changed in response to the injection. Salivary flow rate was measured by weighing swabs inserted into the oral cavity over five minutes. The secondary endpoint was the Drooling Severity and Frequency Scale (DSFS), in which the severity (max. 5 points) and frequency (max. 4 points) of sialorrhea were assessed by the practitioner on two Likert scales. Data from an affiliated extension-period with three additional four-monthly injections in two treatment arms (75 vs. 100 units, no placebo arm), were recently published separately [2].
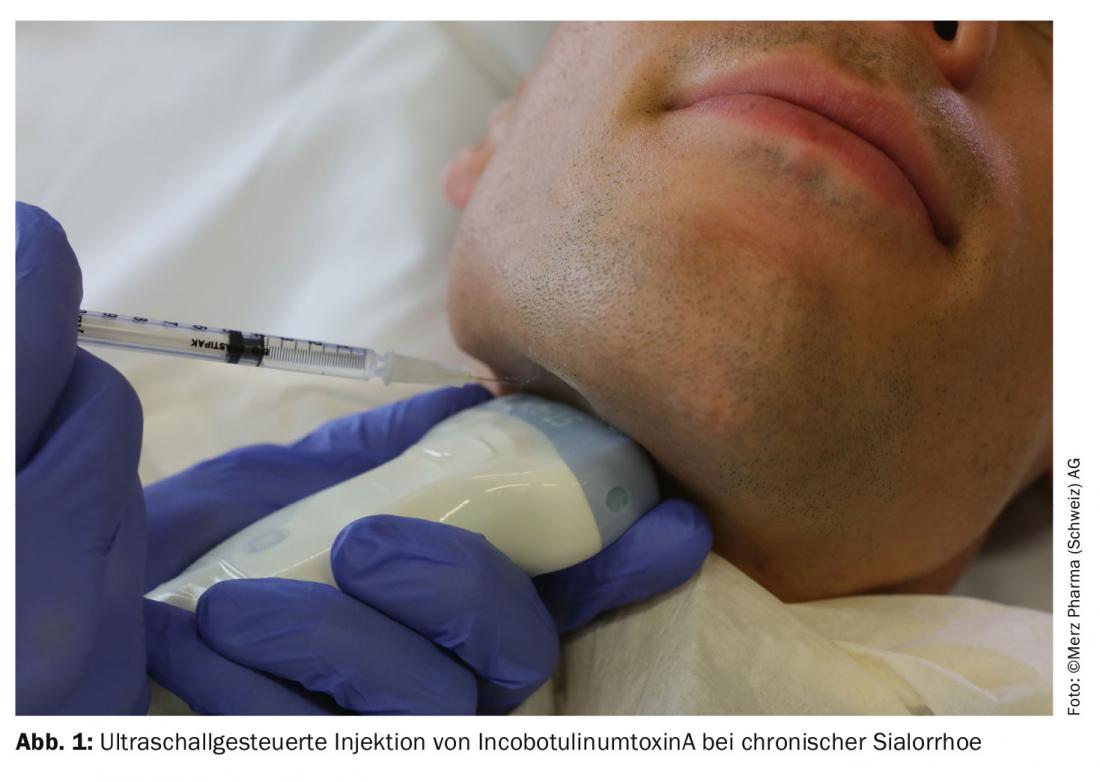
Results
In the 100-unit arm, both co-primary (GICS, p=0.002; uSFR, p=0.004) and secondary endpoints (DSFS, p<0.001) were met. Even 16 weeks after injection, there was still a significant effect both subjectively (GICS, p=0.011) and objectively (uSFR, p=0.002). In the 75-unit arm, only the secondary endpoint (DSFS, p=0.002) was met. However, a secondary analysis at weeks 8 and 12 also showed significant improvement in GICS and uSFR in the 75-unit arm. The choice of injection technique (with ultrasound guidance or based on anatomic landmarks) had no effect on outcome. The most common adverse events in the 100- and 75-unit arms were dry mouth (2.7%, 5.4%, respectively) and dysphagia (0%, 4.1%, respectively).
Conclusion
The study results indicate that incobotulinumtoxinA 100 U appears to be an effective and well-tolerated treatment for chronic sialorrhea in adults.
Comment
The SIAXI study is the largest randomized controlled trial of botulinum toxin treatment of sialorrhea to date. Based on this Class 1 evidence, incobotulinumtoxinA has been approved by the European Medicines Agency (EMA), by the FDA, and in June 2019 by Swissmedic for the treatment of chronic sialorrhea in adults.
IncobotulinumtoxinA is thus the only drug approved in Switzerland for this indication. In addition to this now undoubted proof of efficacy, the study also helps to clarify the long-discussed question of the correct dose. The 100-unit arm showed an earlier and longer-lasting effect compared with the 75-unit arm, with no significantly more frequent side effects. The duration of action of 16 weeks in the 100-unit arm was longer than is usually expected for injections into striated muscle, similar to the long duration of action for sweat gland treatment. Surprisingly, dysphagia occurred only in the 75-unit arm, with two of the three subjects having preexisting mild dysphagia. It must be taken into account that only patients without clinically relevant dysphagia, mapped on the “Modified Radboud Oral Motor inventory for Parkinson’s disease”, were included in the study. Thus, the study does not formally allow conclusions about the safety of treatment for patients with comorbid, higher-grade dysphagia. Likewise, patients with ALS who frequently suffer from sialorrhea were not included in the study. Therefore, botulinum toxin should be used with special caution in these patients. Of note, Swissmedic approval for the treatment of sialorrhea was independent of etiology. Nevertheless, the treatment is only reimbursed in the case of a neurological cause: The FOPH has included the incobotulinumtoxinA indication “sialorrhea” in the list of specialties as of April 1, 2020, with some limitations (see www.spezialitätenliste.ch). Thus, a neurological clarification must be performed first and the treatment may only be performed ultrasound-guided by specialists in neurology or ENT. In particular, the requirement of ultrasound-guided injection seems reasonable. Although no clear conclusions on the superiority of ultrasound-guided injection can be drawn from the SIAXI study, another study was able to show that the hit rate according to anatomical landmarks for Eq. parotis at only 74% and the gl. submandibularis was only 62% [3]. It is hoped that the required use of ultrasound will not result in treatment being offered only at centers. Ultrasound-guided injection is easy to learn with intensive training.
The inclusion of the indication “chronic sialorrhea” in the list of specialties eliminates the time-consuming struggle for approval of costs and thus makes an effective and, under certain conditions, safe therapy for sialorrhea available to our patients sooner.
Literature:
- Ruiz-Roca JA, Pons-Fuster E, Lopez-Jornet P: Effectiveness of the Botulinum Toxin for Treating Sialorrhea in Patients with Parkinson’s Disease: A Systematic Review. Journal of clinical medicine. 2019; 8(3):317.
- Jost WH, Friedman A, Michel O, et al: Long-term incobotulinumtoxinA treatment for chronic sialorrhea: efficacy and safety over 64 weeks. Parkinsonism & related disorders. 2020; 70: 23-30.
- Loens S, Bruggemann N, Steffen A, Baumer T: Localization of Salivary Glands for Botulinum Toxin Treatment: Ultrasound Versus Landmark Guidance. Movement disorders clinical practice. 2020; 7(2): 194-198.
InFo NEUROLOGY & PSYCHIATRY 2020; 18(3): 28-29.