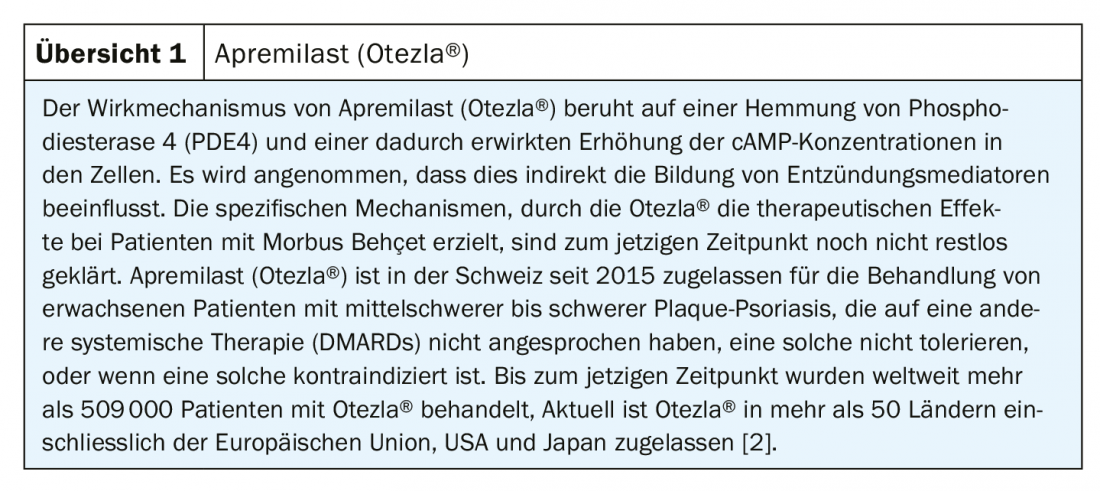
Behçet’s disease is a rare, chronic, multisystemic inflammatory disease that causes vasculitis. Oral aphthae occur in more than 97% of affected individuals and are associated with significant impairment of quality of life. Recently, a new highly effective treatment option has become available in the form of apremilast.
As announced by the pharmaceutical company Amgen in July of this year, Swissmedic approved the extension of marketing authorization for apremilast (Otezla®) [1] for the treatment of persistent oral ulcers associated with Behçet’s disease in adult patients who have responded inadequately to topical therapy [2]. Otezla® is the first and only drug approved by Swissmedic for the treatment of adult patients with oral aphthae associated with Behçet’s disease*. Oral aphthae are the most common manifestation of Behçet’s disease, with over 97% of patients suffering from them. Associated symptoms affect various aspects of life, including food intake and speech. “Recurrent oral aphthae are the most common symptom of patients with Behçet’s disease. The oral aphthous episodes are painful and affect the physical and psychological health of the patients,” explains Prof. Alfred Mahr, MD, Clinic for Rheumatology at the Cantonal Hospital of St. Gallen [2].
* Specific to drug label as of June 2020.
Rapid onset of action and reduction of disease activity
The approval is primarily based on data from the randomized, placebo-controlled, double-blind Phase III RELIEF™ trial (NCT02307513), with additional data from the randomized, placebo-controlled, double-blind Phase II trial (BCT-001) of 111 patients included in the submission [2]. “Today’s approval of the expanded indication is a significant milestone for patients in Switzerland suffering from oral aphthae associated with Behçet’s disease,” commented Thomas Schwaller, MD, Medical Director at Amgen Switzerland AG, on the indication expansion of Otezla®.
The RELIEF™ study evaluated the efficacy and safety of Otezla® in 207 adult patients with Behçet’s disease and active oral aphthae. Study participants had been previously treated with at least one nonbiologic drug and were suitable for systemic therapy. The 64-week study took place at 53 sites in 10 countries [3]. Patients received either Otezla® 30 mg twice daily (n=104) or placebo (n=103) over the 12-week placebo-controlled treatment phase. After completion of week 12, all patients received Otezla® for the 52-week active treatment phase. Efficacy was assessed by the number of oral aphthae and improvement in oral pain [3]. Results from the RELIEF™ study showed that Otezla® 30 mg twice daily resulted in a reduction in oral aphthae compared to placebo at week 12. Otezla® showed a rapid onset of action: The number of oral aphthae and associated pain decreased as early as week 1. In patients who were continuously treated with Otezla® and continued to participate in the study, the improvements persisted until week 64: Reduction of oral aphthae and reduction of oral pain symptoms. Treatment with Otezla® further led to a significant reduction in overall disease activity compared to placebo at week 12, and a significant improvement in the quality of life of Behçet’s disease patients taking Otezla® compared to placebo was derived from a questionnaire [3].
| Behçet’s disease Behçet’s disease, also known as Behçet’s syndrome or Behçet’s disease, is associated with abnormalities of the immune system and inflammation of the blood vessels [4]. Clinical manifestations include recurrent oral or genital ulcers, skin lesions, uveitis, arthritis, and vascular, central nervous system, and gastrointestinal involvement [4]. Oral aphthae may occur in more than 97% of patients with Behçet’s disease [5]. Behçet’s disease has been classified as an orphan disease in many parts of the world. The Middle East, Asia, and Japan have the highest prevalence numbers of Behçet’s disease [6]. |
Tolerability was consistent with the known safety profile of Otezla®. Among the most frequently observed adverse events, which occurred in ≥5% of patients treated with Otezla in the RELIEF™ study, were® treated patients included diarrhea (41.3%), nausea (19.2%), headache (14.4%), upper respiratory tract infection (11.5%), upper abdominal discomfort (8.7%), vomiting (8.7%), and back pain (7.7%) and were mostly mild to moderate in severity [4].
Source: Amgen
Literature:
- Swissmedicinfo: Professional information Otezla® www.swissmedicinfo.ch
- Amgen Switzerland: Treatment of oral aphthae in patients with Behçet’s disease: Amgen Receives Regulatory Extension for Otezla® (Apremilast) in Switzerland, Media Release, Rotkreuz, 6.7.2020.
- Hatemi G, et al: Trial of Apremilast for Oral Ulcers in Behcet’s Syndrome. N Engl J Med 2019;381: 1918-1928, and Hatemi G, et al. Efficacy of Apremilast for Oral Ulcers Associated With Active Behcet’s Syndrome Over 64 Weeks: Results From a Phase III Study). EULAR 2019; Madrid, Spain. Abstract no. OP0146.
- Senusi A, et al: The influence of oral health and psycho-social well-being on clinical outcomes in Behçet’s disease. Rheumatol Int 2018; 38(10): 1873-1883.
- Davatchi F, et al: Behçet’s disease: from east to west. Clin Rheumatol 2010; 29(8): 823-833.
- Leonardo NM, McNeil J: Behçet’s disease: Is there geographical variation? A review far from the silk road. Int J Rheumatol 2015; 2015: 945262.
GP PRACTICE 2020; 15(8): 39
DERMATOLOGY PRACTICE 2020; 30(4): 24











