Inadequately treated arterial hypertension over many years usually leads to heart failure. It is not at all surprising that the onset of heart failure can be delayed by treating blood pressure, obesity, and diabetes.
Inadequately treated arterial hypertension over many years usually leads to heart failure if no other life-limiting disease develops in the meantime. Conversely, patients with heart failure often have arterial hypertension for years beforehand. In the Framingham cohort, 91% of all patients with newly diagnosed heart failure had a prior diagnosis of arterial hypertension over a 20-year follow-up period [1]. The risk of developing heart failure in hypertensive compared with normotensive study participants, adjusted for age and other heart failure precipitating factors, was two times higher for men and three times higher for women. Risk factors for heart failure include myocardial infarction, diabetes mellitus, left ventricular hypertrophy, and valvular disease. The risk of developing heart failure doubles in patients with blood pressure of 160/100 mmHg compared with those with 140/90 mmHg [2].
Thus, it is not at all surprising that the onset of heart failure can be delayed by treating blood pressure, obesity, and diabetes. Men resp. Women without hypertension, obesity, or diabetes at age 45 years live an average of 3 to 15 years longer without the onset of heart failure compared with patients with such risk factors [3]. Normal body weight, no diabetes, and especially absence of arterial hypertension are associated with an 86% lower risk of developing heart failure during life. In the SHEP (Systolic Hypertension in the Elderly Program) study, during an observation period of 4.5 years, life was prolonged by one day per month in patients taking chlorthalidone compared with those taking placebo [4].
Pathophysiology
Diastolic dysfunction is the first manifestation of hypertensive cardiopathy. Cardiac remodeling due to pressure loading (e.g., arterial hypertension, aortic stenosis) results in concentric left ventricular hypertrophy because of Laplace’s law. To compensate for the increased wall stress caused by the pressure, the muscle thickens and the cavum of the ventricle becomes smaller. In contrast, volume loading (e.g., aortic regurgitation, obesity, chronic renal insufficiency, anemia) results in eccentric hypertrophy of the left ventricle (increase in ventricular volume and increased muscle mass) [5].
If the pressure load persists, diastolic dysfunction increases and the concentric hypertrophic left ventricle decompensates, heart failure with preserved left ventricular systolic function (HFpEF – “heart failure with preserved ejection fraction”) occurs. In contrast, if volume loading persists and the left ventricle decompensates, heart failure with reduced left ventricular function (HFrEF) occurs.
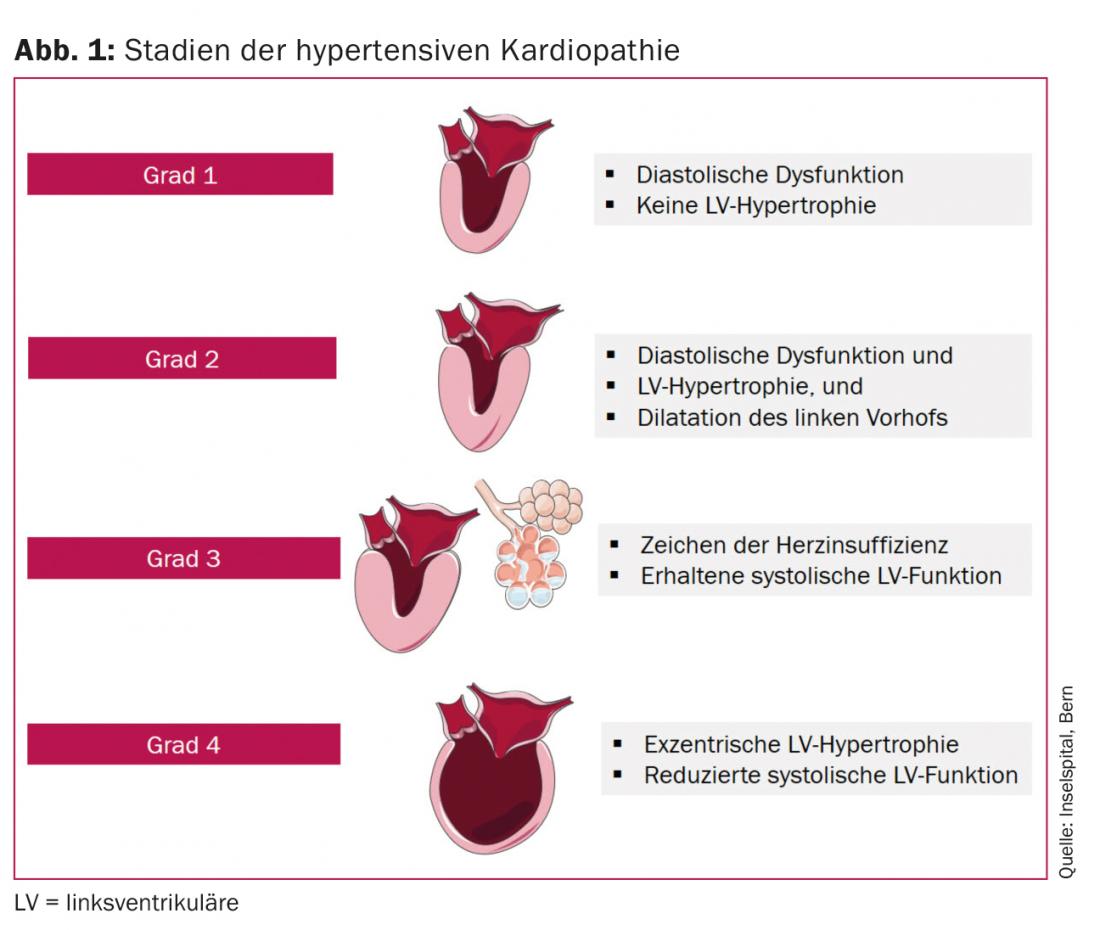
Left ventricular (LV) hypertrophy and elevated biomarkers of subclinical myocardial damage (high-sensitivity troponin, N-terminal pro-B-type natriuretic peptide [NT-pro BNP]) are associated with a higher risk regarding development of symptomatic heart failure, especially HFrEF [6]. In HFpEF, the levels of NT-pro BNP are usually lower, which is explained by lower wall stress and thus fewer circulating natriuretic peptides [7]. Normal natriuretic proteins are found in 30% of patients with HFpEF [8], especially in patients with obesity [9] or with symptoms only under stress [10]. Finally, the end stage of hypertensive cardiopathy, usually the result of years of pressure and volume loading, is dilated cardiopathy. From a clinical point of view, hypertensive cardiopathy can be divided into four stages (Fig. 1):
- Grade 1: Diastolic dysfunction of the left ventricle without LV hypertrophy.
- Grade 2: Diastolic dysfunction of the left ventricle with LV hypertrophy.
- Grade 3: Symptoms of heart failure (dyspnea, pulmonary edema) with preserved LV systolic function.
- Grade 4: Dilated cardiopathy with impaired LV systolic function and symptoms of heart failure [11].
Diastolic dysfunction is the most common effect of long-standing hypertension. However, not all patients with diastolic dysfunction have HFpEF [12], and diastolic dysfunction may be absent altogether in well-treated patients with HFpEF or in patients who present primarily with symptoms on exertion [10,13]. The left atrium is often dilated and systolic pulmonary pressure estimated by Doppler is elevated (>35 mmHg) [14]. Patients with HFpEF show increased LV hypertrophy, lesions in the epicardial coronaries, decrease in coronary microcirculation, and myocardial fibrosis than a control population. The cause of the disruption of coronary microcirculation could be increased systemic inflammation and oxidative stress due to the comorbidities of HFpEF [15,16].
Even isolated diastolic dysfunction can surprisingly cause pulmonary edema, as demonstrated by Gandhi et al. shown [17]. Patients with an episode of hypertensive-induced pulmonary edema showed unchanged normal systolic left ventricular function during and after the episode when blood pressure was well controlled. Mean systolic blood pressure was initially 200 +/- 26 mmHg during pulmonary edema and 139 +/- 17 mmHg at the time of follow-up. Diastolic dysfunction remained as the only cause of pulmonary edema, because transient systolic dysfunction with or without mitral regurgitation was absent in these patients [17].
“Burned out” hypertensive cardiopathy.
In advanced heart failure, systolic blood pressure is often low, even in patients who have always been hypertensive. This phenomenon is referred to as “burned-out” hypertensive cardiopathy. Patients with long-standing hypertension may become increasingly normo to hypotensive as heart failure increases, i.e., LV systolic function decreases. Severely impaired LV systolic function results in decreased cardiac output, and compensatory mechanisms such as peripheral vasoconstriction fail to preserve cardiac output and thus fail to arrest the fall in blood pressure. Patients with “burned-out” hypertensive cardiopathy poorly tolerate blood pressure-lowering medications such as angiotensin receptor blockers (ARBs), angiontensin-converting enzyme (ACE) inhibitors, beta blockers (BBs), and diuretics.
The interplay of high blood pressure, hypertensive cardiopathy, and dilated cardiopathy (as a sign of “burned-out” hypertensive cardiopathy, i.e., the end stage of hypertensive cardiopathy) is complex. Often, it is only retrospectively revealed that arterial hypertension was (partly) causative for dilated cardiopathy. If LV systolic function recovers, blood pressure also rises again.
Arterial hypertension in patients with HFrEF.
Although arterial hypertension is a known risk factor for heart failure, high systolic blood pressure appears to be associated with lower mortality in HFrEF patients. Several studies showed improved outcome of high systolic blood pressure in patients with acute as well as chronic heart failure [18–24]. Thus, even in 2289 patients in the COPERNICUS (Carvedilol Prospective Randomized Cumulative Survival) trial, lower systolic blood pressure was associated with a higher risk of death [25]. Part of the reason for the positive effect of beta-blocker therapy on survival in heart failure may be the increase in central blood pressure. Accordingly, the decrease in heart rate with beta-blocker therapy (which is indirectly proportional to the increase in central pressure) is associated with better survival in patients with heart failure in the SHEP study [26]. The same phenomenon may apply to ivabradine, where a reduction in hospitalizations for heart failure was achieved in the SHIFT study (“Systolic Heart failure treatment with the If inhibitor ivabradine”) [27]. In contrast to the normal hypertensive patient, where a reduction in heart rate is associated with increased cardiovascular mortality, the opposite is true in patients with heart failure [26,28].
Cardiorenal syndrome
End-organ damage occurs in both the heart and kidney with long-standing arterial hypertension. Thus, not only cardiac but also renal insufficiency occurs in these patients. Interaction between the heart and kidney occurs in both directions via a wide variety of mechanisms [29]. The most common cardiorenal syndromes in heart failure patients are the following [30]:
- Type 1 (acute): Acute heart failure leads to acute kidney damage (renal failure).
- Type 2 (chronic): Chronic heart failure leads to increasing disease of the kidney
- Type 3: Acute deterioration of kidney function leads to heart failure
- Type 4: Occurrence of heart failure due to increasing underlying renal disease.
From the clinician’s perspective, the presence of renal failure complicates heart failure therapy. As renal failure progresses, there is increased heart failure, as well as vice versa. Corresponding comorbidities and many heart failure medications increase the risk for hyperkalemia. A pronounced cardiorenal syndrome drastically limits the choice of medication and its up-dosing to the target dose. A combination of newly explored K+ binders (eg, patiromer) and a mineral corticoid antagonist may in the future reduce cardiovascular mortality and morbidity in patients with heart failure [31].
Pickering Syndrome
In 1988, Dr. Pickering et al. reported. on eleven patients with hypertension and bilateral atherosclerotic renal artery stenosis who presented with multiple episodes of sudden onset pulmonary edema [32]. Pickering syndrome, sudden onset pulmonary edema (“flash pulmonary edema”) and bilateral renal artery stenosis, is a type 3 cardiorenal syndrome. Patients with Pickering syndrome usually present with diastolic dysfunction and LV hypertrophy; systolic LV function is normal or only mildly impaired. This, together with insufficient natriuresis due to bilateral renal artery stenosis, is the mechanism leading to pulmonary edema (Fig. 2) . The fact that this pulmonary edema occurs suddenly and leads to a life-threatening emergency distinguishes Pickering syndrome from other forms of cardiac decompensation [33]. Recurrent sudden pulmonary edema, lack of typical angina, elevated blood pressure, and elevated creatinine levels should suggest bilateral renal artery stenosis, ie, Pickering syndrome, as the cause of sudden pulmonary edema. In the Pickering syndrome case series, there has usually been a preceding episode of multiple sudden onset pulmonary edemas until the diagnosis of bilateral renal artery stenosis was made [32].
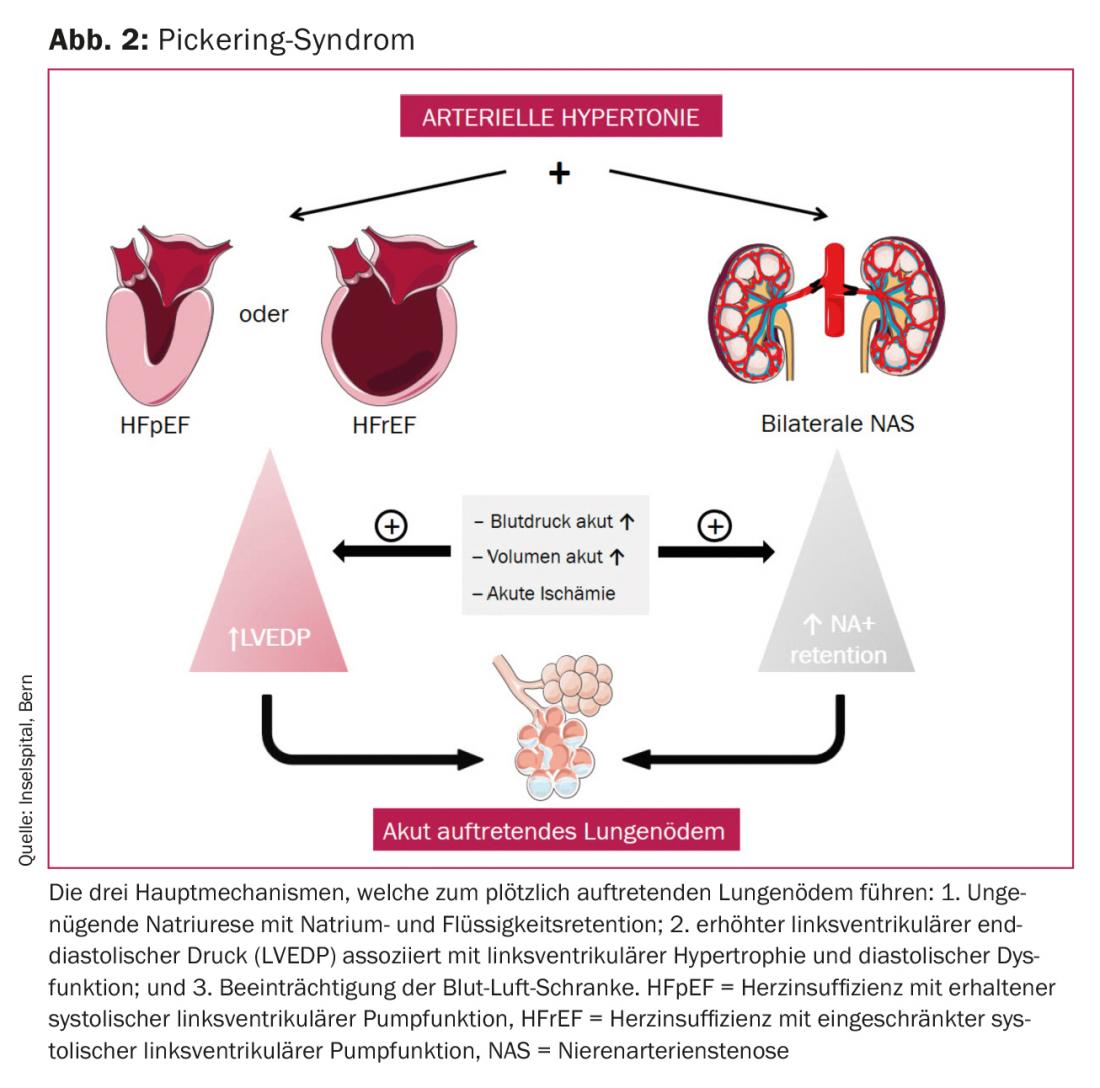
The goal of acute treatment of sudden-onset pulmonary edema is to achieve sufficient oxygenation by lowering afterload to decrease postcapillary wedge pressure and maintaining sufficient diuresis. Correcting the cause, i.e., angioplasty of the renal artery stenosis, is essential once the patient has been stabilized.
Antihypertensive therapy to reduce the incidence of heart failure.
By definition, all antihypertensive drugs reduce blood pressure. But, looking closely at the literature, not all drugs are equally effective in reducing the incidence of heart failure. Beta-blockers are a cornerstone of therapy for heart failure with reduced LV systolic function (HFrEF) [34]. However, compared to the other antihypertensive drugs, they do not have a better preventive character regarding heart failure. In 112 177 patients from 12 different randomized controlled trials, therapy with BB probably resulted in a reduction in blood pressure of 12.6/6.1 mmHg compared with placebo. However, no significant reduction in the incidence of heart failure was found [35]. Compared with other antihypertensive drugs, BB do not add any effect in terms of reduction of all-cause mortality, cardiovascular mortality, or myocardial infarction. However, there is an increase in stroke prevalence in elderly patients. Therefore, BB should not be the first choice of antihypertensive therapy.
In a Cochrane meta-analysis, calcium channel blockers (CCBs) increased the risk of heart failure (risk ratio [RR]: 1.37; 95% confidence interval (CI): 1.25-1.51) compared with diuretics. Although CCBs reduce the risk of stroke compared with ACE inhibitors and reduce the risk of myocardial infarction and stroke compared with ARBs, there appears to be increased heart failure with CCBs compared with ACE inhibitors (RR 1.16; 95% CI 1.06-1.27) and also compared with ARBs (RR 1.2, 95% CI: 1.06-1.36) [36]. However, a recent meta-analysis questions this: in it, blood pressure reduction with CCB seems to prevent the occurrence of heart failure as efficiently as blood pressure reduction with other drugs [37]. Thus, the previously demonstrated inferiority of CCB may have resulted from better concomitant medication in the control arm.
In the ALLHAT (Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial) study, stroke and cardiovascular disease occurred more frequently with the alpha-blocker doxazosin compared with chlorthalidone. The risk of heart failure with doxazosin therapy was twice as high (RR 2.04, 95% CI 1.79-2.32, p>0.001) [35]. Thus, it appears that treatment with alpha blockers should be avoided in patients at risk for heart failure.
Blockade of the renin-angiotensin system efficiently lowers blood pressure and reduces the incidence of heart failure. ACE inhibitors are as effective as ARBs [38,39]. Here, particular reference should be made to the newly approved ARB/neprilysin inhibitor valsartan/sacubitril, which is not only a potent drug in the treatment of heart failure with reduced LV systolic function but is also likely to lower blood pressure well [40]. In the PARADIGM (Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure) trial, valsartan/sacubitril showed a resounding reduction in cardiovascular mortality and morbidity in patients with reduced LV systolic function [41]. However, it remains to be seen whether valsartan/sacubitril has a good risk-benefit ratio in the long term in hypertensive patients.
Last but not least, thiazide-like diuretics such as chlorthalidone or indapamide are an excellent choice of antihypertensive medication for the prevention of heart failure. In the SHEP-[42] as well as in the HYVET (Hypertension in the Very Elderly Trial) study [43], these resulted in a significant reduction in heart failure compared with placebo, for chlorthalidone (RR 0.51; 95% CI 0.37-0.71) and for indapamide (RR 0.36; 95% CI 0.22-0.58; p<0.001). Thiazide-like diuretics have been shown to be superior to all other antihypertensive drugs with respect to prevention of heart failure in 10 randomized controlled trials (RR 0.84; 95% CI 0.73-0.98) [37]. However, such results do not exist for hydrochlorothiazide, either for heart failure or for any other cardiovascular end point. Thus, unlike indapamide or chlorthalidone, hydrochlorothiazide should be avoided in hypertensive patients at risk for heart failure.
In the TOPCAT (Treatment of Preserved Cardiac function Heart Failure with an Aldosterone Antagonist) trial, in patients with HFpEF, the aldosterone antagonist spirinolactone had no effect on a composite end point of cardiovascular death or hospitalization [44]. However, spirinolactone therapy resulted in a reduction in the number of hospitalizations due to heart failure, but also in an increase in renal failure and hyperkalemia. However, a post-hoc analysis with only patients from the North American continent shows a positive composite endpoint [45]. Surprisingly, increased endpoints occurred in Georgia and Russia in the intervention group compared with the control group. Then looking in the blood for metabolites of spirinolactone, it was undetectable in 30% of patients in Russia who were supposed to take the drug, compared to 3% in North America [46].
In summary, most antihypertensive drug classes slow the transition from hypertension to heart failure, although not all classes are equally efficient in this regard. Once-daily hydrochlorothiazide should be avoided, especially since two very good alternatives are available in chlorthalidione and indapamide.
Antihypertensive drugs in patients with heart failure and continued high blood pressure levels.
Usually, low blood pressure is the more common problem in patients with heart failure [47]. However, sometimes persistent hypertension occurs in patients with HFpEF, less commonly in HFrEF. Due to a lack of evidence, our recommendations are empirical, based on clinical and pathophysiologic considerations [48]. These recommendations apply to patients who continue to have elevated blood pressure values (>140/90 mmHg in the office/hospital) despite therapy with off-dose ACE inhibitor or ARB, beta blocker, and diuretic.
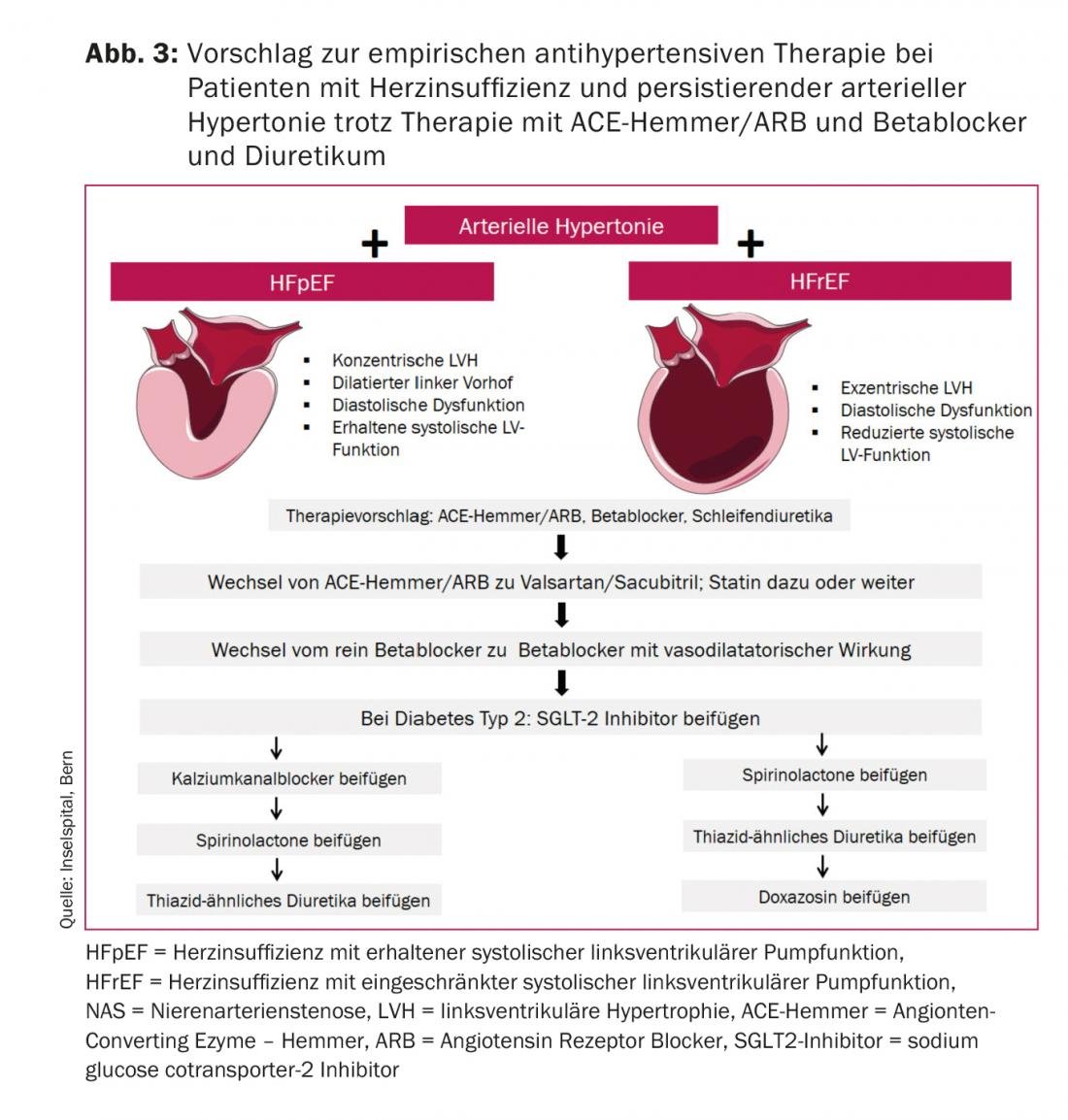
The first thing that should always be checked is therapy adherence. The goal of further expansion of therapy after beta blockers, ACE inhibitors or ARBs, and loop diuretics is to improve diastolic and microvascular dysfunction in HFpEF and to preserve or possibly improve impaired systolic left ventricular function in HFrEF. Figure 3 shows our recommendation with initial increased afterload lowering with valsartan/sacubitril instead of the ACE inhibitor/ARB and a switch to a vasodilator beta blocker such as carvedilol or nebivolol. Also, a statin should be started in patients with HFpEF and continued high BP.
Take-Home Messages
- 90% of all patients with newly diagnosed heart failure had prior arterial hypertension.
- Diastolic dysfunction, left ventricular hypertrophy and dilatation of the left atrium are the signs of hypertensive cardiopathy.
- Normal natriuretic peptides are found in 30% of heart failure patients with preserved systolic function, especially in obese patients.
- In dilated cardiopathy (in the setting of “burned-out” hypertensive cardiopathy), it is often only retrospectively apparent after recovery of LV systolic function that hypertension was causative.
- ACE inhibitors, angiotensin receptor blockers, and indapamide or chlorthalidone are the antihypertensive drugs of choice to prevent heart failure.
Literature:
- Levy D, Larson MG, et al: The progression from hypertension to congestive heart failure. JAMA 1996; 275: 1557-1562.
- Lloyd-Jones DM, Larson MG, et al: Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation 2002; 106: 3068-3072.
- Ahmad FS, Ning H, et al: Hypertension, Obesity, Diabetes, and Heart Failure-Free Survival: The Cardiovascular Disease Lifetime Risk Pooling Project. JACC Heart Fail 2016; 4: 911-919.
- Kostis JB, Cabrera J, et al: Association between chlorothalidone treatment of systolic hypertension and long-term survival. JAMA 2011; 306: 2588-2593.
- Messerli FH: Cardiovascular effects of obesity and hypertension. Lancet 1982; 1: 1165-1168.
- Seliger SL, de Lemos J, et al: Older Adults, “Malignant” Left Ventricular Hypertrophy, and Associated Cardiac-Specific Biomarker Phenotypes to Identify the Differential Risk of New-Onset Reduced Versus Preserved Ejection Fraction Heart Failure: CHS (Cardiovascular Health Study). JACC Heart Fail 2015; 3: 445-455.
- Iwanaga Y, Nishi I, et al: B-type natriuretic peptide strongly reflects diastolic wall stress in patients with chronic heart failure: comparison between systolic and diastolic heart failure. J Am Coll Cardiol 2006; 47: 742-748.
- Anjan VY, Loftus TM, et al: Prevalence, clinical phenotype, and outcomes associated with normal B-type natriuretic peptide levels in heart failure with preserved ejection fraction. Am J Cardiol 2012; 110: 870-876.
- Bishu K, Deswal A, et al: Biomarkers in acutely decompensated heart failure with preserved or reduced ejection fraction. Am Heart J 2012; 164: 763-770 e3.
- Borlaug BA, Nishimura RA et al: Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail 2010; 3: 588-595.
- Iriarte M, Murga N, et al: Classification of hypertensive cardiomyopathy. Eur Heart J 1993; 14 Suppl J: 95-101.
- Redfield MM, Jacobsen SJ et al: Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA 2003; 289: 194-202.
- Franssen C, Paulus WJ: Normal resting pulmonary artery wedge pressure: a diagnostic trap for heart failure with preserved ejection fraction. Eur J Heart Fail 2015; 17: 132-134.
- Thenappan T, Prins KW, et al: Pulmonary hypertension secondary to heart failure with preserved ejection fraction. Can J Cardiol 2015; 31: 430-439.
- Paulus WJ, Tschope C: A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013; 62: 263-271.
- Mohammed SF, Hussain S, et al: Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation 2015; 131: 550-559.
- Gandhi SK, Powers JC, et al: The pathogenesis of acute pulmonary edema associated with hypertension. N Engl J Med 2001; 344: 17-22.
- Adamopoulos C, Zannad F, et al: Ejection fraction and blood pressure are important and interactive predictors of 4-week mortality in severe acute heart failure. Eur J Heart Fail 2007; 9: 935-941.
- Bhatia RS, Tu JV, et al: Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med 2006; 355: 260-269.
- Fonarow GC, Adams KF, et al: Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA 2005; 293: 572-580.
- Gheorghiade M, Abraham WT, et al: Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. JAMA 2006; 296: 2217-2226.
- Lee TT, Chen J, Cohen DJ, Tsao L: The association between blood pressure and mortality in patients with heart failure. Am Heart J 2006; 151: 76-83.
- Pulignano G, Del Sindaco D, et al: Clinical features and outcomes of elderly outpatients with heart failure followed up in hospital cardiology units: data from a large nationwide cardiology database (IN-CHF Registry). Am Heart J 2002; 143: 45-55.
- Schrier RW, Abraham WT: Hormones and hemodynamics in heart failure. N Engl J Med 1999; 341: 577-585.
- Rouleau JL, Roecker EB, et al: Influence of pretreatment systolic blood pressure on the effect of carvedilol in patients with severe chronic heart failure: the Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) study. J Am Coll Cardiol 2004; 43: 1423-1429.
- McAlister FA, Wiebe N, et al: Meta-analysis: beta-blocker dose, heart rate reduction, and death in patients with heart failure. Ann Intern Med 2009; 150: 784-794.
- Swedberg K, Komajda M, et al: Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet 2010; 376: 875-885.
- Rimoldi SF, Messerli FH, et al: Selective Heart Rate Reduction With Ivabradine Increases Central Blood Pressure in Stable Coronary Artery Disease. Hypertension 2016; 67: 1205-1210.
- Bock JS, Gottlieb SS: Cardiorenal syndrome: new perspectives. Circulation 2010; 121: 2592-2600.
- Ronco C, Cicoira M, McCullough PA: Cardiorenal syndrome type 1: pathophysiological crosstalk leading to combined heart and kidney dysfunction in the setting of acutely decompensated heart failure. J Am Coll Cardiol 2012; 60: 1031-1042.
- Epstein M, Pitt BC: A propitious time for initiating clinical trials in patients with heart failure with reduced ejection fraction and an estimated glomerular filtration rate <30 mL/min with a mineralocorticoid receptor antagonist and a K+ binder: “the forbidden fruit”. Eur Heart J 2016; 37: 3130-3134.
- Pickering TG, Herman L, et al: Recurrent pulmonary oedema in hypertension due to bilateral renal artery stenosis: treatment by angioplasty or surgical revascularisation. Lancet 1988; 2: 551-552.
- Rimoldi SF, Yuzefpolskaya M, et al: Flash pulmonary edema. Prog Cardiovasc Dis 2009; 52: 249-259.
- Kotecha D, Manzano L, et al: Effect of age and sex on efficacy and tolerability of beta blockers in patients with heart failure with reduced ejection fraction: individual patient data meta-analysis. BMJ 2016; 353: i1855.
- Bangalore S, Wild D, et al: Beta-blockers for primary prevention of heart failure in patients with hypertension insights from a meta-analysis. J Am Coll Cardiol 2008; 52: 1062-1072.
- Chen N, Zhou M, et al: Calcium channel blockers versus other classes of drugs for hypertension. Cochrane Database Syst Rev 2010: CD003654.
- Thomopoulos C, Parati G, Zanchetti A: Effects of blood pressure-lowering treatment. 6. prevention of heart failure and new-onset heart failure – meta-analyses of randomized trials. J Hypertens 2016; 34: 373-384; discussion 84.
- Bangalore S, Fakheri R, et al: Angiotensin-Converting Enzyme Inhibitors or Angiotensin Receptor Blockers in Patients Without Heart Failure? Insights From 254,301 Patients From Randomized Trials. Mayo Clin Proc 2016; 91: 51-60.
- Messerli FH, Bangalore S: Angiotensin Receptor Blockers Reduce Cardiovascular Events, Including the Risk of Myocardial Infarction. Circulation 2017; 135: 2085-2087.
- Bavishi C, Messerli FH, et al: Role of neprilysin inhibitor combinations in hypertension: insights from hypertension and heart failure trials. Eur Heart J 2015; 36: 1967-1973.
- McMurray JJ, Packer M, et al: Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371: 993-1004.
- SHEP Cooperative Research Group: Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA 1991; 265: 3255-3264.
- Beckett NS, Peters R, et al: Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358: 1887-1898.
- Pfeffer MA, Pitt B, McKinlay SM: Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014; 371: 181-182.
- Pfeffer MA, Claggett B, et al: Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation 2015; 131: 34-42.
- de Denus S, O’Meara E, et al: Spironolactone Metabolites in TOPCAT – New Insights into Regional Variation. N Engl J Med 2017; 376: 1690-1692.
- Redfield MM: Heart failure with preserved ejection fraction. N Eng J Med 2016; 375: 1868-1877.
- Messerli FH, Rimoldi SF, Bangalore S: The Transition from Hypertension to Heart Failure: Contemporary Update. JACC Heart Fail 2017;5: 541-551.
CARDIOVASC 2017; 16(5): 30-35











