Especially in the field of medical image recognition, artificial intelligence (AI) applications are widely developed, which is a very interesting application area for dermatology. For an adequate implementation of digital innovations, regulations by health authorities and recommendations in guidelines are important regulatory instruments. There is a lot going on in this regard at the moment.
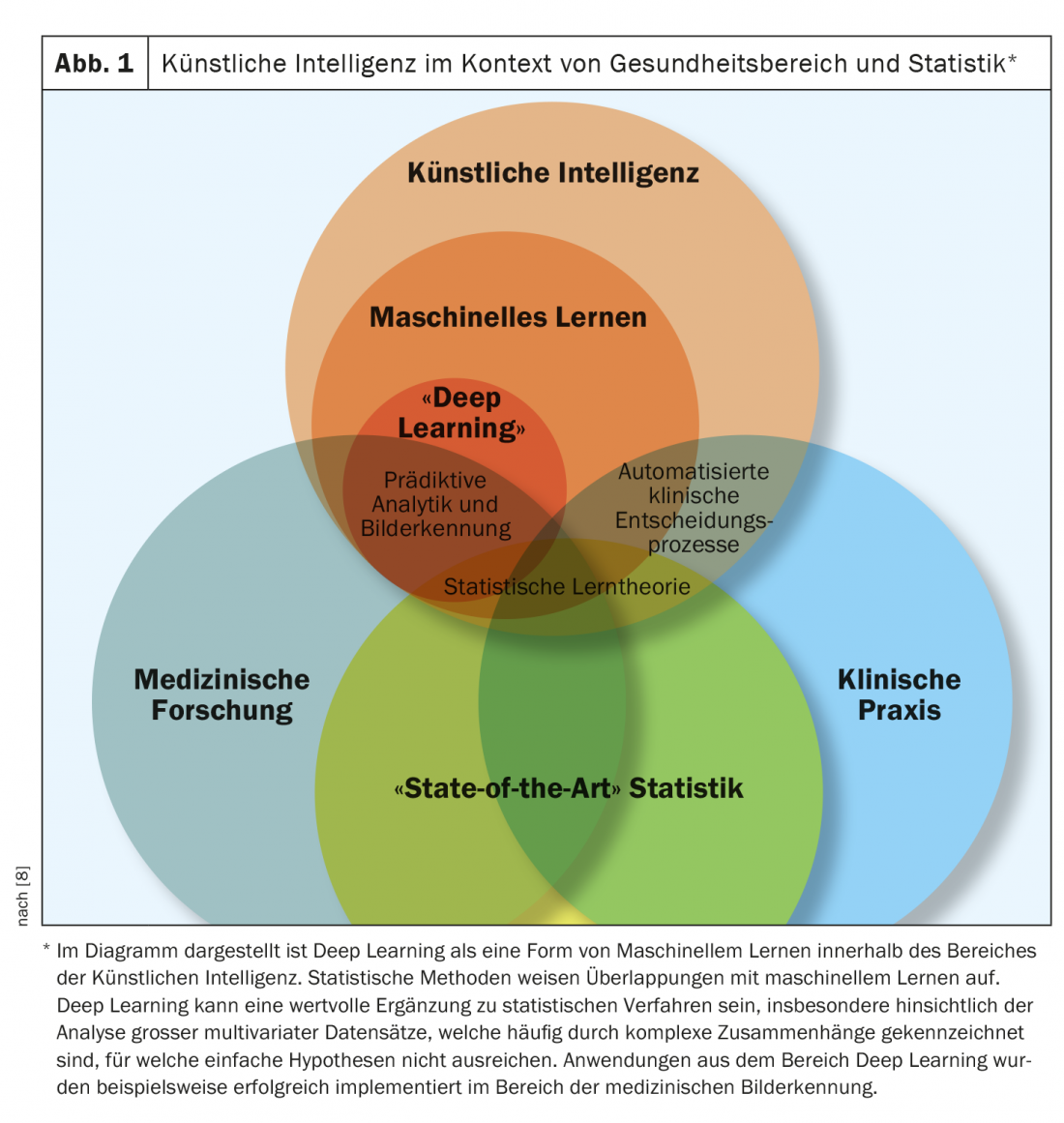
Artificial intelligence (AI) is increasingly being used in medicine to support human expertise, is very topical in COVID-19 times, and is expected to become increasingly important in the future (Fig. 1). With AI technologies, computers can be trained to process large amounts of data and recognize specific patterns in the data (Overview 1). The potential of these applications as well as possible dangers in the interaction between human and artificial intelligence are the subject of numerous current studies. At this year’s virtual EADV Annual Congress, international experts summarized current findings in this field.
Opportunities and risks of digital applications in the healthcare sector
The fact that applications based on artificial intelligence (AI) in the healthcare sector, in addition to certain risks, also hold many opportunities and can contribute to saving resources was demonstrated by engineer and entrepreneur Jacques Biot, former president of the École Polytechnique, Paris, in his presentation [1]. In view of growing data volumes, solutions for efficient data management are gaining in importance and can be an important element of personalized medicine, concludes a publication published this year by Matheney et al. [2]. In the context of implementing digital technologies in healthcare, regulations are an important tool. A lot has happened in the U.S. in this area recently. In April 2018, the U.S. Food and Drug Administration (FDA) approved a medical device that uses AI algorithms for early detection of retinopathy. It is the first FDA approval of a device that can be used independent of a physician’s evaluation [3]. Furthermore, a specific regulatory framework for the implementation of AI methods in medical software (“software as a medical device”, SaMD) has been developed by the FDA as part of the “Digital Health Innovation Action Plan” [4]. The translation of scientific findings into clinical applications, including certification by health authorities, is one of the major challenges in the field of digital health. In summary, AI applications will play an increasingly important role in precision medicine, optimizing resource utilization, and enabling individuals to access healthcare services regardless of location. Multidisciplinary collaboration is essential for the development and implementation of viable digital applications, including regulation by health authorities and other entities.

AI-assisted skin cancer diagnostics
Josep Malvehy, MD, Department of Dermatology, University Hospital of Barcelona [5], spoke on the use of digital applications in the diagnosis of pigmented lesions. The fact that support from an AI tool can increase the accuracy of diagnostic assessment is demonstrated by the results of an experiment led by MedUni Vienna published in Nature this year [6]. In this study, 302 physicians were presented with dermatoscopic images of benign and malignant skin lesions, first without and then with the support of artificial intelligence, for evaluation. There were three variants: In the first case, the AI showed the examiner the probabilities of all possible diagnoses, in the second case the probability of a malignant change, and in the third case a selection of similar images with known diagnoses, comparable to an image search via Google. It turned out that in the first case, collaboration with AI improved the diagnostic accuracy of the investigators, by over 10%. These results confirm the findings of previous studies. In of a study by Tschandl et al. published last year, in which 511 human physicians competed against 139 image recognition algorithms (77 different laboratories worldwide) for the assessment of pigmented lesions as part of the ISIC Challenge of the International Skin Imaging Collaboration [7]. An image database containing over 10,000 light microscopy images with seven different classes of pigmented skin lesions served as the learning basis for the machines. In the experiment, all participants were randomly presented with 30 images each on an online platform from a pool of new photos not available in the image database. While the image recognition programs achieved slightly better results, they still cannot replace humans with their current capabilities, the authors concluded, because diagnosing a patient also involves observing the patient’s progress and interpreting it in the overall context. But these two studies show the potential of AI applications as a supporting tool in the field of skin cancer diagnostics. In conclusion, the speaker again emphasizes the importance of regulations for the development and use of apps in the “real world” setting (Box).

Source: EADV 2020
Literature:
- Biot J: Consequences of deep learning for the healthcare system. Jacques Biot, Paris. Artificial intelligence and big data, EADV Vienna (Virtual), 29.10.2020
- Matheny ME, et al: Artificial Intelligence in Health Care. A Report From the National Academy of Medicine. JAMA 2020; 323(6): 509-510.
- U.S. Food & Drug Administration: FDA permits marketing of artificial intelligence-based device to detect certain diabetes-related eye problems, www.fda.gov/news-event. Last call 02.11.2020
- U.S. Food & Drug Administration: Software as a Medical Device (SaMD), www.fda.gov/medical-devices/digital-health-center-excellence
- Malvehy J : Diagnosis of pigmented lesions. Josep Malvehy, MD, Artificial intelligence and big data, EADV Vienna (Virtual), Oct. 29, 2020.
- Tschandl P, et al: Human-computer collaboration for skin cancer recognition. Nature Medicine 2020; 26: 1229-1234.
- Tschandl P, et al: Comparison of the accuracy of human readers versus machine-learning algorithms for pigmented skin lesion classification: an open, web-based, international, diagnostic study. Lancet Oncol 2019; 20(7): 938-947.
- Krittanawong C, et al: Deep learning for cardiovascular medicine: a practical primer. Eur Heart J 2019; 40(25): 2058-2073.
- Keutzer L, Simonsson USH: Medical Device Apps: An Introduction to Regulatory Affairs for Developers. JMIR Mhealth Uhealth 2020; 8(6): e17567.
- Medical Device Regulation (MDR [EU]) 2017/745, https://eur-lex.europa.eu, last accessed 02.11.2020
- Digital Switzerland, www.digitalerdialog.ch/de/neue-schwerpunkte-fur-die-digitale-schweiz, last accessed 02.11.2020
- Interpharma, www.interpharma.ch/themen/fuhrend-in-forschung-entwicklung/mit-hochwertigen-gesundheitsdaten-medizinischen-fortschritt-sichern/kuenstliche-intelligenz-ki/, last accessed 02.11.2020
DERMATOLOGIE PRAXIS 2020; 30(6): 55-56 (published 9/12/20, ahead of print).