Chronic metabolic acidosis is a common complication in renal failure patients. The disease is associated with accelerated progression of chronic kidney disease and increased all-cause mortality. Acidosis correction via diet, oral bicarbonate substitution and, if necessary, adjustment of dialysis therapy are some of the possible measures.
The homeostasis of pH, the acid-base balance, is an important regulatory goal of the organism and is one of the most complex tasks of the kidney. However, with increasing renal function impairment, it is subject to functional disturbances. To balance the daily acid load, the kidney not only reabsorbs bicarbonate, but also excretes ammonium. This capacity decreases with progression of renal insufficiency, especially if the filtration capacity is <40 ml/min. Due to the reduced ammonio-genesis, the excretion capacity for fixed acids decreases and the reabsorption or synthesis of bicarbonate is also reduced.
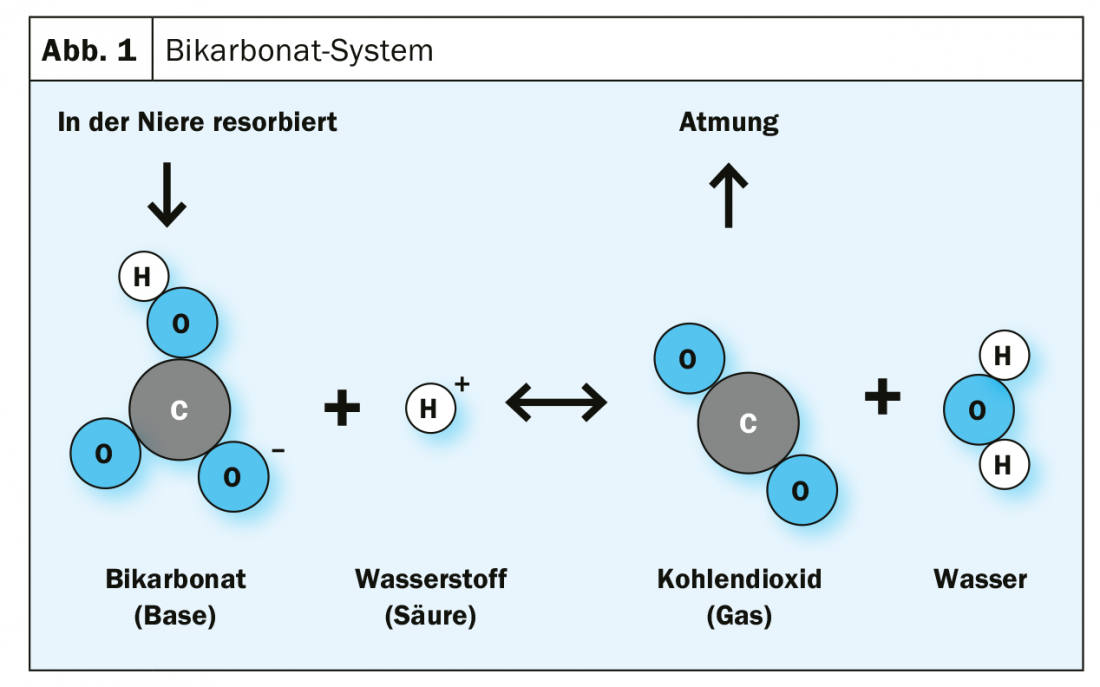
Every day, the human metabolism thus produces about 12,000 mmol of acid equivalents from the breakdown of organic acids, proteins and phospholipids. Normally, the bicarbonate ion (HCO3-) decomposes into carbon dioxide (CO2) and water (H2O) after binding an acid equivalent (proton, H+). Carbon dioxide is then exhaled through the lungs to dispose of acid equivalents (Fig. 1) [1]. If kidney function declines, not enough hydrogen carbonate ions are available for this process. In a large cohort, metabolic acidosis was observed in 13% of CKD patients with stage III or higher chronic kidney disease, and in 37% of patients with stage IV CKD [1]. In dialysis patients (stage V), the proportion is as high as >60% [2].
Definition metabolic acidosis
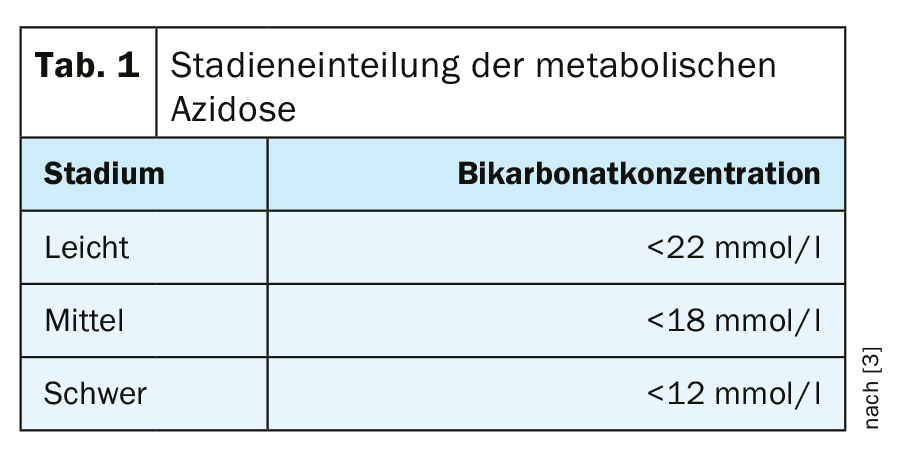
Chronic metabolic acidosis is said to occur when the pH is below 7.34 and the serum carbonate level has dropped to below 22 mmol/l with normal or only slightly decreased pCO2 values in blood gas analysis [3] (Table 1). In most cases, the disease is asymptomatic, but may result in very unpleasant dyspnea. Long-term symptoms include muscle wasting, increased fracture risk, increased incidence of hyperkalemia, and more rapid progression of renal failure.
Especially in the case of impaired renal function, the disturbances of bone metabolism and the development of renal osteopathy are caused by altered vitamin D metabolism and secondary hyperparathyroidism. However, metabolic acidosis aggravates renal osteopathy, among others. Buffering of the increased H+ ions results in increased calcium loss from bone tissue. In addition, increased phosphaturia leads to hypophosphatemia, which stimulates bone resorption. Impairment of the parathyroid glands also promotes parathyroid hormone secretion.
The extent of acidosis can vary considerably between individual patients. In diabetic patients, for example, less pronounced forms are often found than in non-diabetic patients with comparable renal insufficiency [4].
Diagnostics
Initial clues to the genesis are provided by a physical examination, medical history, and past medical history. Questions that should be focused on include whether intoxications, infections, or gastrointestinal symptoms may be present. Furthermore, the presence of pre-existing conditions and the use of medication should be checked.
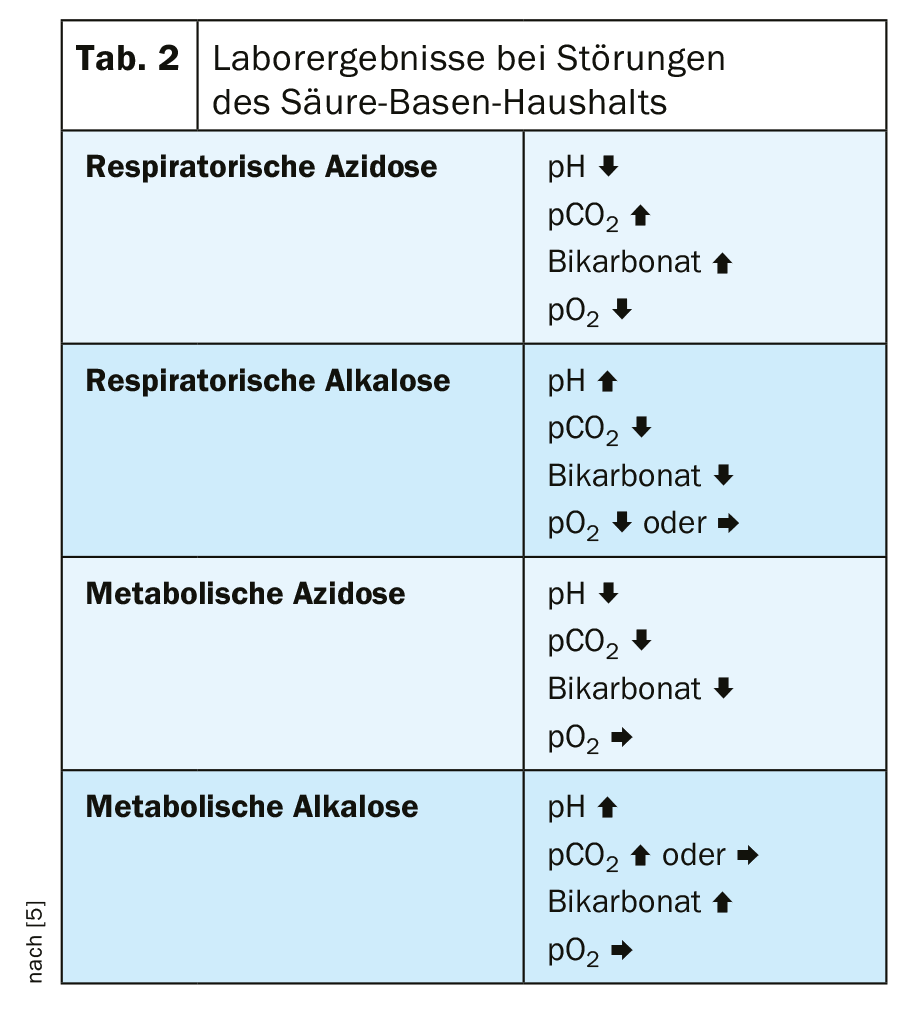
Venous determination of pH, pCO2, and bicarbonate concentration is usually used to assess metabolic acidosis. With lowered pH and bicarbonate, pCO2 is usually also lowered due to compensatory increased expiration. Differential diagnosis should exclude respiratory acidosis, respiratory alkalosis, and metabolic alkalosis (Table 2) [5].
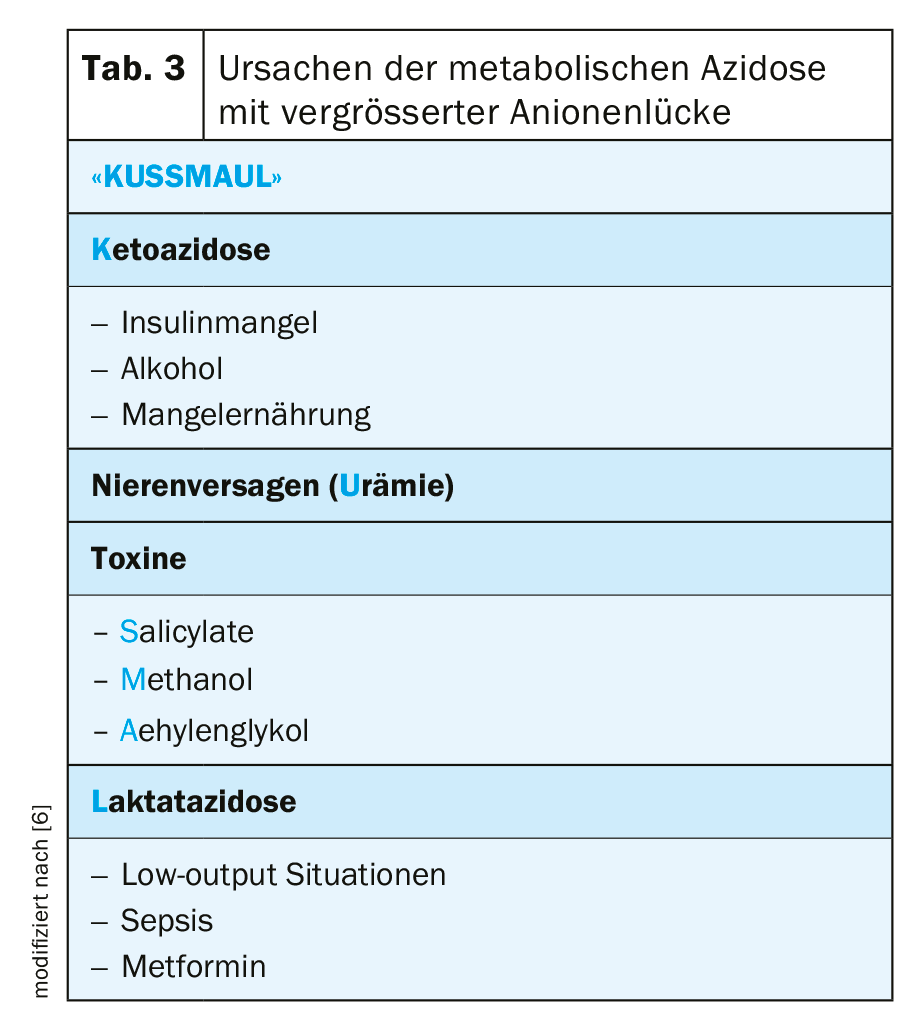
However, it is also important to distinguish between hyperchloremic acidosis and acidosis with a large anion gap, because in plasma the absolute amount of cations and anions is always balanced. The calculation of the anion gap (= sodium – [Chlorid + aktuelles Bikarbonat]) allows a differentiated procedure (Fig. 2) . The standard value of the anion gap is bi 12 ± 2 mmol/l. An anion gap >25 mmol/l practically always indicates organic acidosis, such as lactate or ketoacidosis. A large anion gap also indicates poisoning with aspirin, ethylene glycol, or rarely methanol. Clinically conspicuous in this case is a pronounced Kussmaul’s respiration (tab. 3).
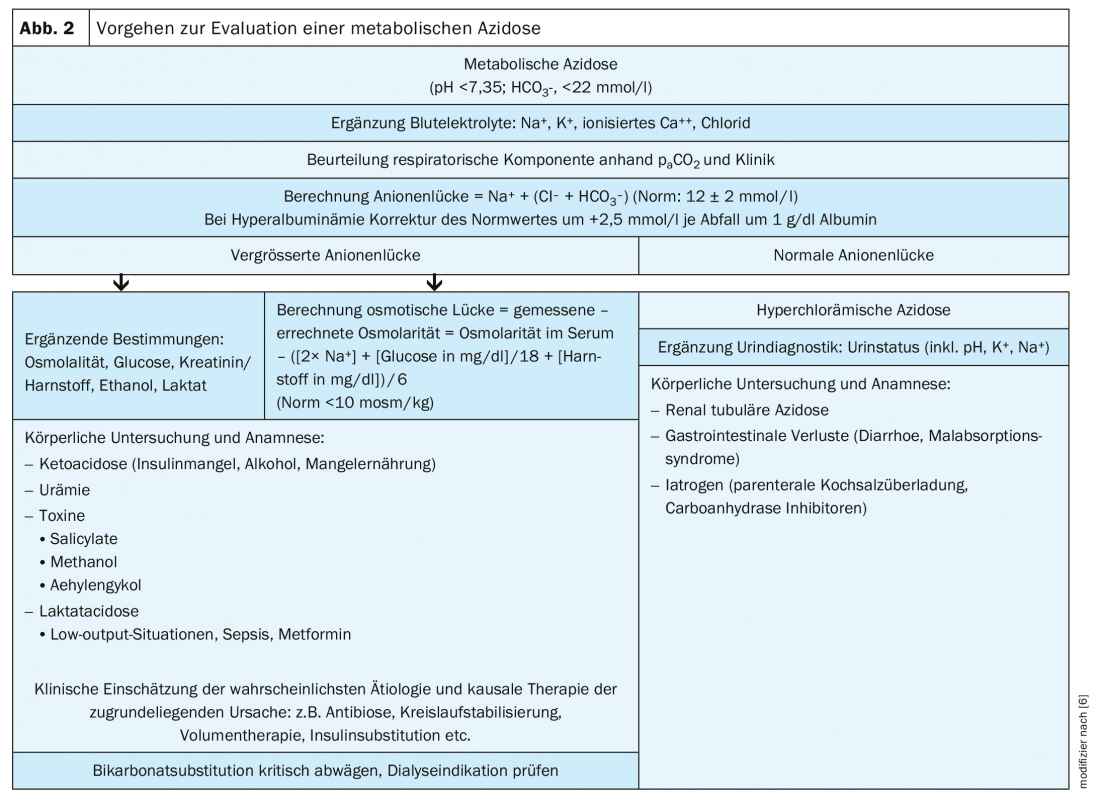
Especially in chronic renal insufficiency, the accumulation of sulfates and other acids in the context of uremia can lead to metabolic acidosis with a slightly increased anion gap. In acute renal failure, on the other hand, severe acidosis rarely occurs-except, for example, in the intensive care unit when there is a surge of metabolites in highly catabolic states [7].
Accelerated progression of renal failure
It is now believed that metabolic acidosis, which is indeed an expression of renal dysfunction, has an impact on the progression of renal insufficiency. As early as CKD stage II, retention of acids leads to elevated endothelin and aldosterone levels. These then induce deterioration of renal function via increased vasoconstrictor effects and favoring fibrotic remodeling processes [8,9]. A retrospective cohort observational study of approximately 5400 adult patients with chronic renal failure demonstrated that progression of renal disease is accelerated by acidosis [10]. Patients who had bicarbonate levels as high as 22 mmol/l at baseline were more likely to reach a renal endpoint with doubling of serum creatinine or renal failure requiring dialysis. The decreased serum bicarbonate level was associated with accelerated progression of renal disease.
Therapy
The treatment of metabolic acidosis is based on two principles: The first is the reduction of dietary acid load through dietary changes. However, this requires a reduction in protein intake, which increases the risk of muscle breakdown. Ultimately, however, a change in diet should be recommended even in patients with moderate renal function impairment – if there is evidence of metabolic acidosis. The diet in Western industrialized countries has too high a protein content and therefore leads to an increased generation of acid valencies and too low a proportion of alkali components. To achieve a moderate protein intake, the amount of fresh fruits and vegetables should be increased.
The second principle is to increase the intake of alkaline substances, whether through diet or alkalinizing therapy. Sodium bicarbonate (Nephrotrans®) is the most commonly used. Here it is recommended to administer 2 to a maximum of 3 times daily 4-9 capsules of 500 mg. However, calcium carbonate or calcium or potassium citrate can also be administered, especially if hyperphosphatemia is present. However, care must be taken to avoid side effects. In rare cases, blood pressure may increase, edema and digestive disorders may occur.
Sodium bicarbonate substitution
Therefore, if dietary measures are not sufficient to reach the threshold value of 22 mmol/l, sodium bicarbonate should be substituted in particular. An open-label, controlled study was conducted to evaluate the effect of sodium bicarbonate therapy on creatinine doubling in patients with stage 3-5 chronic renal insufficiency (CNI) and metabolic acidosis compared with standard care over 36 months [11]. A 1:1 ratio was used to randomize 795 patients. SB was administered twice daily to achieve a target serum bicarbonate concentration of 24-28 mmol/l. The initial daily dose was calculated to replace half of the bicarbonate deficit. Subsequently, this was increased by 25% weekly until the serum bicarbonate target was reached. Sodium bicarbonate dose was adjusted to maintain serum bicarbonate levels as needed <28 mmol/l. In the standard care group, rescue treatment with sodium bicarbonate was not protocolized and was allowed at the discretion of the treating physician for safety reasons.
Participants received nutritional counseling and a diet low in protein (0.8 or 0.6 or 0.3 g/kg/day, depending on the severity of CNI), low in phosphorus (<800 mg/day), and controlled in sodium (<7 g/day) at the discretion of the treating physician. The goals were:
- BP <135/90 mmHg
- Serum hemoglobin 11-12 g/dL
- Low Density Lipoprotein (LDL) Cholesterol <100 mg/dl
- Serum phosphorus 2.7-4.6 mg/dl
- Serum calcium 8.4-10.2 mg/dl
- Serum parathyroid hormone (PTH) 70-150 pg/ml and
- Serum 25 (OH) vitamin D 30-100 nmol/l.
The primary endpoint was time to doubling of serum creatinine. All-cause mortality and time to initiation of renal replacement therapy were secondary end points. The effect of sodium bicarbonate on blood pressure, nutritional parameters, fluid retention, and hospitalization was also evaluated as a safety endpoint.
Preserve kidney function
A total of 87 participants met the primary endpoint [62 (17.0%) in standard care versus 25 (6.6%) with sodium bicarbonate, p<0.001]. Cox proportional hazard analysis showed that participants treated with sodium bicarbonate had a 64% lower hazard rate for doubling of serum creatinine during follow-up (HR 0.36; 95% CI 0.22-0.58; p<0.001) (Fig. 3). Similarly, 71 participants (45 [12,3%] in standard care and 26 [6,9%] with sodium bicarbonate, p=0.016) started dialysis, while 37 participants (25 [6,8%] in standard care and 12 [3,1%] with sodium bicarbonate, p=0.004) died. Survival analysis showed that participants treated with sodium bicarbonate had a 57% lower HR of all-cause mortality during follow-up. There were no significant effects of SB on blood pressure, total body weight, though there were in terms of hospitalizations. In conclusion, in individuals with CNI 3-5 without advanced stages of chronic heart failure, treatment of metabolic acidosis with sodium bicarbonate is safe and improves renal and patient survival. Untreated participants had a threefold higher risk of creatinine doubling or a twofold higher risk of starting dialysis. It is also significant that the effect of sodium bicarbonate on creatinine clearance was maintained regardless of baseline serum bicarbonate levels, baseline renal function, age, sex, systolic blood pressure, and proteinuria.
Take-Home Messages
- Chronic metabolic acidosis is a common complication in renal failure patients.
- In the presence of metabolic acidosis, the anion gap should always be calculated.
- The disease is associated with accelerated progression of chronic renal failure and increased all-cause mortality.
- Metabolic acidosis requires acidosis correction via diet, oral bicarbonate substitution, and adjustment of dialysis therapy if necessary.
- Sodium bicarbonate substitution has been shown to be safe and effective in terms of renal and patient survival.
Literature:
- Pathophysiology, diagnosis and therapy of chronic metabolic acidosis in renal failure. Diabetes aktuell 2014; 12(3): 110-112.
- Raphael KL, et al: Prevalence of and risk factors for reduced serum bicarbonate in chronic kidney disease. Nephrology 2014; 19: 648-654.
- Hoyer J: Chronic metabolic acidosis in renal failure. Nephrologist 2012; 7: 472-480.
- Caravaca F, Aprobas M, Pizarro JL, Esparrago JF: Metabolic acidosis in advanced renal failure: differences between diabetic and nondiabetic patients. Am J Kidney Dis 1999; 33: 892-898.
- Kosch M, Schaefer RM: Disorders of the acid-base balance. Rational diagnostics and economic therapy. Dtsch Ärztebl 2005; 102(26): A1896-1899.
- Schricker S, Schanz M, Alscher MD, Kimmel M: Metabolic acidosis. Diagnostics and therapy. Med Clin Intensivmed Notfmed 2019; doi.org/10.1007/s00063-019-0538-y.
- Kraut JA, Madias NE: Metabolic acidosis of CKD: an update. Am J Kidney Dis 2016; 67: 307-317.
- Wesson DE, Simoni J, Broglio K, Sheather S: Acid retention accompanies reduced GFR in humans and increases plasma levels of endothelin and aldosterone. Am J Physiol Renal Physiol 2011; 300: F830-F837.
- Kovesdy CP: Metabolic acidosis and kidney disease: does bicarbonate therapy slow the progression of CKD? Nephrol Dial Transplant 2012; 27(8): 3056-3062.
- Shah SN, Abramowitz M, Hostetter TH, Melamed ML: Serum bicarbonate levels and the progression of kidney disease: a cohort study. Am J Kidney Dis 2009; 54(2): 270-277.
- DI Iorio BR, Bellasi A, Raphael KL, et al: Treatment of metabolic acidosis with sodium bicarbonate delays progression of chronic kidney disease: the UBI Study. J Nephrol 2019; 32(6): 989-1001.
FAMILY PRACTICE 2020; 15(6): 6-9
This educational text was produced with the kind support of Salmon Pharma.