Today, fluticasone furoate is the most potent inhaled corticosteroid (ICS) with the highest therapeutic index available to date [2,3]. It is a component of Relvar Ellipta along with vilanterol. Daley-Yates et al. showed that fluticasone furoate provided 3.4-fold greater protection with fewer systemic effects than other ICS [2,3]. Similarly, the Salford Lung Study confirmed that 25% more patients achieved relevant asthma control with Relvar Ellipta than with other controllers [1]. We talked about this with Professor Dr. Laurent Nicod, Hirslanden Private Clinic, Lausanne, and Dr. Jean-Luc Kurzen, Senior Physician, Pneumology, Männedorf Hospital.
The term “asthma control” is used so naturally today as if everyone meant the same thing – far from it. Not all physicians and patients understand the same thing by asthma control [4]. Many physicians even overestimate the extent of their patients’ asthma control, as one study showed [5]. This was not true for about half of the asthmatics classified as controlled [5]. Patients also often think they have their asthma under control by adapting their lifestyle to their symptom burden [4]. They avoid physical activity, suffer daytime symptoms and nighttime awakenings due to shortness of breath and/or cough, and depend on their emergency medication [4]. De facto, up to 71% of patients do not achieve asthma control despite treatment [1]. This underscores the importance of proactive asthma control that avoids symptoms and thus improves quality of life [6].
Proactive instead of symptom-oriented
If therapy is based only on current symptoms, the goal of good asthma control will be missed [7]. In the MART
*
– schemes, symptom control is based on patient perception, and this often leaves much to be desired [4,7]. In contrast to the MART, the proactive therapy with Relvar Ellipta treated symptoms as well as chronic inflammation, airway remodeling, and bronchial hyperresponsiveness [8].
Relvar Ellipta increases the number of symptom-free days and nights compared with fluticasone furoate or fluticasone propionate, among others [9]. Patients can benefit from this proactive asthma management by being able to resume their daily activities, which Prof. Nicod also points out in the interview [9].
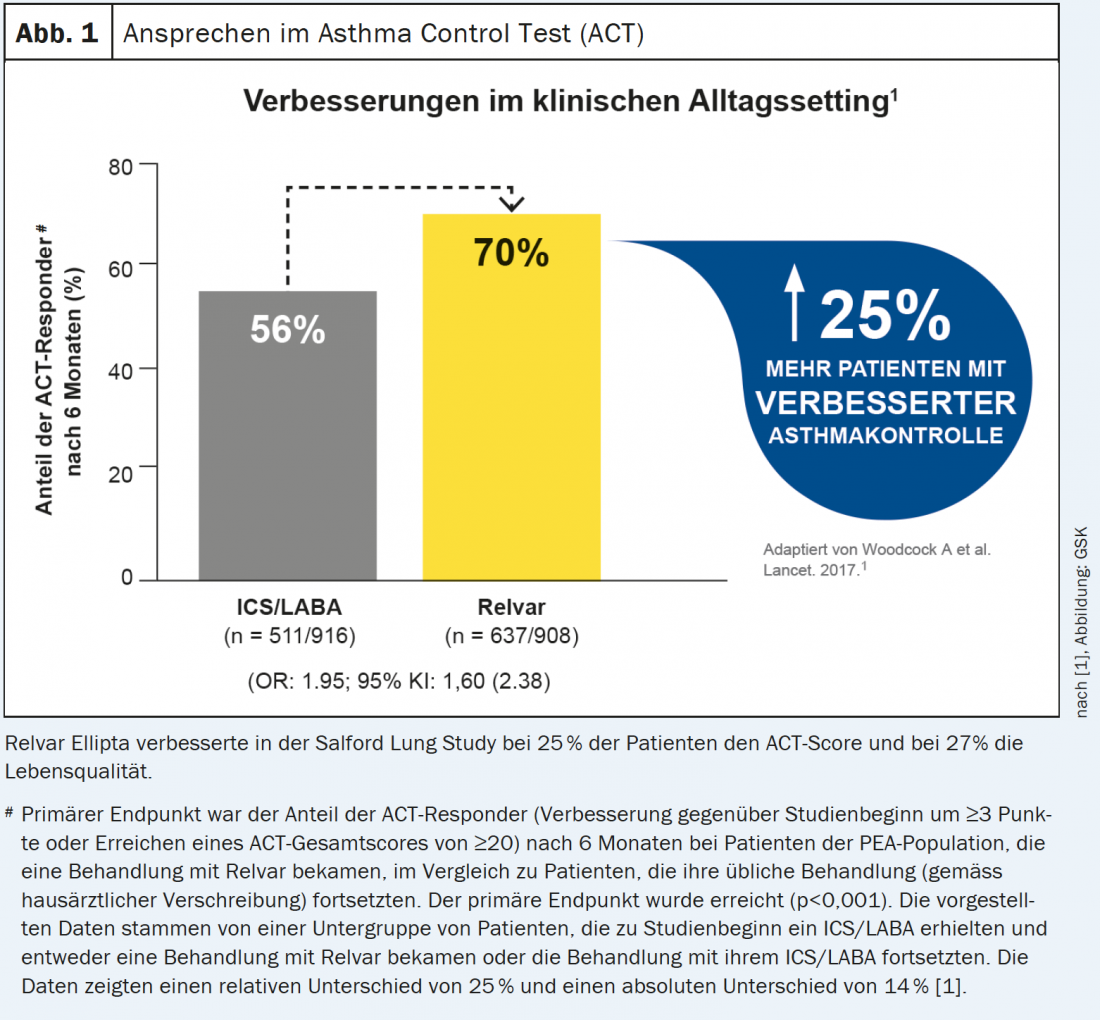
Salford Lung Study is a milestone study [1].
The Salford Lung Study, a real- life setting study, enrolled 4233 patients with inadequately controlled asthma and randomized them to treatment for 52 weeks with either Relvar Ellipta or the previous therapy (ICS or ICS/LABA), which could be optimized as needed [1]. Primary endpoint: achievement of an ACT (Asthma Control Test) score >20 or improvement in score by ≥3 points at 24 weeks [1].
Results
- Proactive asthma control led to improved control in 25% more patients (56% in the control group vs. 70% under Relvar Ellipta) in daily practice [1] (Fig. 1).
- In addition to symptoms, quality of life also improved in 27% more patients [1,10].
- The adverse event profile in both study arms was comparable, with pneumonia occurring infrequently [1].
High therapeutic index as a milestone
Fluticasone furoate in Relvar has a significantly higher therapeutic index than other ICSs, and therefore a stronger anti-inflammatory effect can be achieved with less systemic exposure [2,3]. Thus, at the lowest dosage, it shows a 3.4-fold higher anti-inflammatory effect than budesonide [2,3]. Relvar in the Ellipta device, when used once daily, provides added value that patients feel immediately, namely airway inflammation decreases [8], bronchial hyperresponsiveness decreases [8], and remodeling in the airways is slowed [8].
Quality of life improved
The noticeable effect, which manifests itself in an improvement of all components of the ACT, is offset by only very minor adverse systemic effects [10]. In particular, patients benefited from a relevantly improved quality of life [10].



Imprint
Text:
Dr. Renate Weber
Initial publication:
Medical Tribune 40/2021. The interview partners agree with the second publication. ©Medical Tribune
This article was produced with the kind support of GlaxoSmithKline AG, Talstrasse 3-5, 3053 Münchenbuchsee, Switzerland.
PM-CH-FFV-ADVR-210003-11/2021
© Prime Public Media AG, Zurich 2021
Literature:
1. Woodcock A, et al: Effectiveness of fluticasone furoate plus vilanterol on asthma control in clinical practice: an open-label, parallel-group, randomised controlled trial. Lancet 2017; 390: 2247-2255.
2. Daley-Yates P: Inhaled corticosteroids: potency, dose equivalence and therapeutic index. BJCP 2015; 80(3): 372-380.
3. Daley-Yates P, et al: Therapeutic index of inhaled corticosteroids in asthma: A dose-response comparison on airway hyperresponsiveness and adrenal axis suppression. BJCP 2021; 87: 483-493.
4 Fletcher M, Hiles D: Continuing discrepancy between patient perception of asthma control and real world-symptoms: a quantitative online survey of 1,083 adults with asthma from the UK. Prim Care Respir J 2013; 22: 431-438.
Greenblatt M, et al: Comparison of physician and patient assessment of asthma control. Respiratory Medicine 2010; 104(3); 356-361.
6. Correira de Sousa J, et al: Asthma control, quality of life, and the role of patient enablement: a cross-sectional observational study. Prim Care Respir J 2013; 22(2): 181-187.
7 Chapman KR, et al: Single maintenance and reliever therapy (SMART) of asthma: a critical appraisal. Thorax 2021; 65: 47-52.
8. Ishmael FT: The inflammatory response in the pathogenesis of asthma. The Journal of the American Osteopathic Association 2011; 111: S11-S17.
9. Kerwin E, et al: Fluticasone furoate/vilanterol once daily improves nighttime awakenings in asthma patients with night symptoms: post-hoc analysis of three randomized controlled trials. J Asthma 2018; 55: 890-897.
10. Svedsater H, et al: Patient-reported outcomes with initiation of flutucasone furoate/vilanterol versus continuing usual care in the Salford Lung Study. Respir Med 2018; 141: 198-206.
11. Katsaounou P, et al: Omalizumab as alternative to chronic use of oral corticosteroids in severe asthma. Respir. Med. 2019; 150: 51-62.
12. Wells KE, et al: Real-world effects of once vs. greater daily inhaled corticosteroid dosing on medication adherence. Ann. Allergy, Asthma Immunol. 2013; 111: 216-220.
13 Molimard M, et al: Chronic obstructive pulmonary disease exacerbation and inhaler device handling: real-life assessment of 2935 patients. Eur Respir J 2017; 49(2): 1601794.
Relvar Ellipta (single-dose powder for inhalation). W: Fluticasone furoate 92 or 184 μg, vilanterol 22 μg. I: Bronchial asthma: regular treatment in adults and adolescents 12 years and older if symptomatic on ICS and short-acting bronchodilator. COPD: symptomatic treatment in patients with FEV.
1
<70% and ≥2 exacerbations in the past 12 months. D: Bronchial asthma: adults and adolescents 12 years and older: 1× tgl. 1 inhalation of Relvar Ellipta 92/22 or 184/22. COPD: adults 40 years and older: 1× daily. 1 inhalation Relvar Ellipta 92/22. Relvar Ellipta 184/22 is not approved for COPD.
AI:
Hypersensitivity to any ingredient, severe milk protein allergy. W/V: Not for the treatment of acute asthma symptoms or acute COPD exacerbations. If paradoxical bronchospasm occurs, treat immediately with short-acting bronchodilator, discontinue Relvar Ellipta, consider other therapies. Cardiovascular effects such as arrhythmias possible; prior to therapy, clarification regarding concomitant cardiovascular diseases (including ECG recommended for clarification of QTc prolongation). Caution with diabetes, pulmonary tuberculosis, chronic/untreated infections. Relvar Ellipta 184/22 should not be used in moderately or severely impaired liver function. Systemic adverse effects may occur at high doses for prolonged periods. Visual disturbances may occur with systemic and topical use of corticosteroids, and referral of the patient to an ophthalmologist for evaluation of possible causes should be considered. COPD patients on Relvar Ellipta experienced increased cases of pneumonia. In asthma patients, pneumonia occurred more frequently with Relvar Ellipta 184/22 than with Relvar Ellipta 92/22 or placebo. IA: Caution with concomitant administration of β-blockers, as well as drugs that prolong QTc interval, have sympathomimetic effects, or affect potassium levels. Concomitant administration of potent CYP3A4 inhibitors (e.g., ketoconazole, itraconazole, clarithromycin, ritonavir, or products containing cobicistat) should be avoided unless benefit outweighs increased risk of systemic corticosteroid side effects; then patients should be monitored for systemic corticosteroid side effects. S/S: Pregnancy: Relvar Ellipta should not be used during pregnancy unless clearly necessary. Breastfeeding: discontinue breastfeeding or treatment with Relvar Ellipta. UW: Very common: headache, nasopharyngitis. Common: pneumonia, upper respiratory tract infections, bronchitis, flu symptoms, pain or candidiasis in the mouth and throat, sinusitis, pharyngitis, rhinitis, cough, hoarseness, abdominal, joint, back pain, fractures, pyrexia. Occasionally: extrasystoles. Post-marketing experience: common: muscle spasms; occasional: palpitations, tachycardia, hyperglycemia; rare/unknown : Hypersensitivity reactions (including anaphylaxis, angioedema, urticaria, rash), tremor, anxiety, paradoxical bronchospasm. AK: B, cash-approved. Status of information: January 2019. GlaxoSmithKline AG, 3053 Münchenbuchsee. Detailed information can be found at www.swissmedicinfo. ch. Please report adverse drug reactions at pv.swiss@gsk.com. Professionals can request the above references from GlaxoSmithKline AG.