Treatment with statins is part of the standard therapy for high cholesterol levels. However, a substantial proportion of patients on statin therapy alone do not achieve the LDL-C levels specified by the European Society of Cardiology (ESC). The active substance bempedoic acid, which was approved a few months ago, closes this treatment gap. Significant lipid lowering can be achieved by this well-tolerated add-on therapy option, as the current study situation shows.
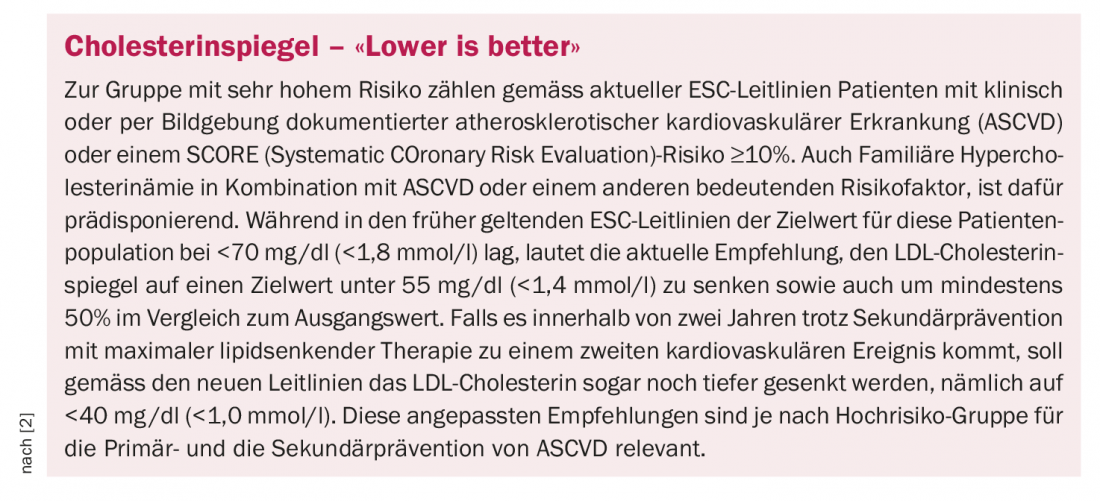
That high LDL cholesterol (LDL-C) levels are a causal risk factor for atherosclerotic cardiovascular events (ASCVD) such as myocardial infarction, heart failure, stroke, and peripheral arterial disease is considered scientifically proven [1]. The benefits of cholesterol lowering for primary and secondary prevention have been documented in numerous randomized-controlled clinical trials. The dose-response relationship between extent of LDL cholesterol lowering and reduction in cardiovascular events was underlying for the risk-based LDL-C levels in the current ESC guidelines [2].
Supply reality shows need for therapy alternatives
In everyday clinical practice, however, the corresponding ESC target values are only partially achieved. Data from Germany show that only in a minority of high-risk patients LDL-C is below 70 mg/dl and only in about one in ten a value <55 mg /dl was recorded [2–4]. Results of a large retrospective Swiss observational study paint a similar picture: of 11,779 patients on statin therapy, 59% had a high or very high cardiovascular risk [11]. However, only 44% of patients achieved the target LDL-C levels specified by the ESC.
One of the reasons for this “gap” in the reality of care is that a dose increase of statins is associated with only modest lipid-lowering effects. “There is the famous ‘Rule of Six’ which says that no matter what statin dose you start at: If you double the dose from 20 mg /d to 40 mg /d or from 40 mg /d to 80 mg /d, you can only achieve about 6% more cholesterol reduction,” explains PD Christian Marc Schmied, MD, of the University Hospital Zurich [5]. This indicates that alternative treatment strategies are needed to achieve the target values proposed by the ESC.
New lipid-lowering agent revolutionizes treatment options
Bempedoic acid addresses this supply gap in the area of lipid lowering. The two orally administered drugs Nilemdo® (bempedoic acid) and the combination drug Nustendi® (bempedoic acid and ezetimibe) have been approved in Switzerland since December 2020 for patients whose elevated cholesterol levels cannot be adequately lowered by drugs such as statins [6]. Both drugs, as add-ons to a statin or other lipid-lowering therapies, produce a significant reduction in LDL-C (Fig. 1).
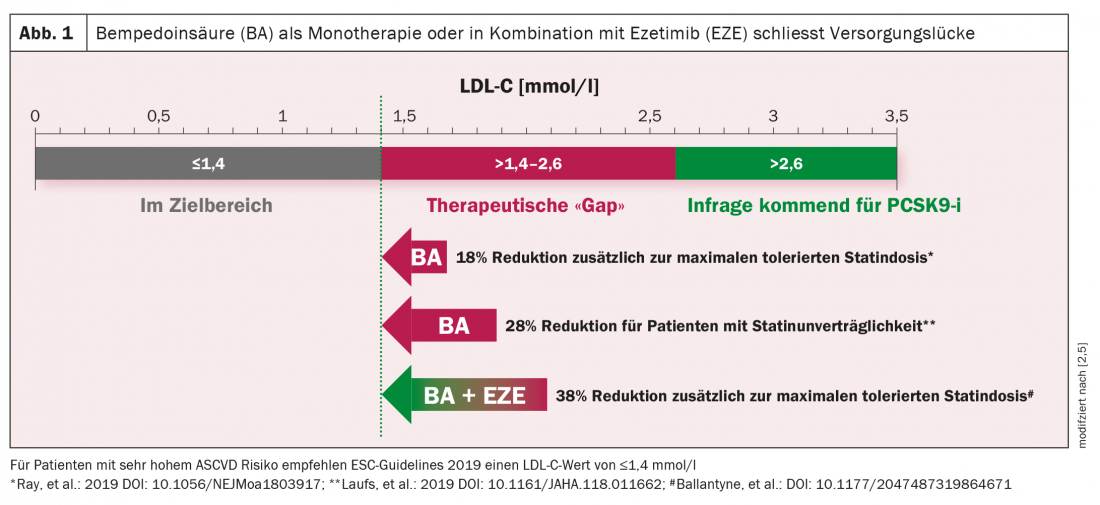
The Swissmedic approval is based on data from the CLEAR (“Cholesterol Lowering via Bempedoic acid, an ACL-Inhibiting Regimen”) study program [7]. In the randomized double-blinded CLEAR SERENITY trial, 345 female and male patients with elevated LDL cholesterol levels were included, with a mean age of 65.2 years [7]. Baseline LDL-C levels in study participants averaged 157.6 md/dland 93% reported statin-associated muscle pain. Subjects received either 180 mg bempedoic acid orally once daily or placebo for 24 weeks. In addition, they were able to continue an already existing lipid-lowering baseline therapy, for example with cholesterol resorption inhibitors. After 12 weeks, treatment with bempedoic acid significantly reduced LDL cholesterol by 21.4% compared with placebo [95% CI, -25.1% to -17.7%]; p <0,001). A significant reduction in non-HDL C (“non-high-density lipoprotein cholesterol”) was also observed, namely by 17.9% compared to placebo. Total cholesterol decreased by 14.8%, apolipoprotein B by 15%, and high-sensitivity C-reactive protein by 24.3% (each p<0.001). Bempedoic acid proved to be well tolerated. Myalgia occurred in 4.7% of patients in the treatment arm and in 7.2% of the placebo group.
To investigate the effects of bempedoic acid on cardiovascular morbidity and mortality, the CLEAR outcomes trial is currently ongoing, with results expected in 2022 [8]. Approximately 12,600 patients with manifest atherosclerosis and a statin intolerance are participating.
Good tolerability thanks to innovative mechanism of action
Statin-associated muscle pain is a common problem affecting up to 10% of patients [9]. For these, bempedoic acid represents an important additional therapeutic option, since bempedoic acid is applied as a prodrug and is activated in the liver by a specific enzyme not expressed in skeletal muscle. Bempedoic acid causes a decrease in the production of cholesterol in the liver by inhibiting ATP citrate lyase, which simultaneously leads to a decrease in LDL cholesterol levels in the blood. The study program on bempedoic acid specifically included and investigated the complex group of patients with statin-associated muscular complaints [10]. CLEAR SERENITY and CLEAR TRANQUILITY demonstrate good tolerability of bempedoic acid in this patient population [1].
In summary, bempedoic acid represents an important add-on therapeutic option for patients with unmet LDL cholesterol targets, including those with statin-associated muscle symptoms. In addition to adequate pharmacotherapy, stress reduction, avoidance of physical inactivity, and abstinence from nicotine are also important aspects. This is because cardiovascular risk can be cumulatively reduced the more factors are controlled, Dr. Schmied emphasized [5].
Congress: College of Family Medicine 2021
Literature:
- Joint statement on the benefit assessment according to § 35a SGB V of bempedoic acid NILEMDO® and bempedoic acid/ezetimibe NUSTENDI®, Last updates 01.02.2021.
- Mach F, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2019;41(1): 111-188.
- Katzmann JL, et al: Non-statin lipid-lowering therapy over time in very-high-risk patients: effectiveness of fixed-dose statin/ezetimibe compared to separate pill combination on LDL-C. Clin Res Cardiol 2020.
- Fox KM, et al: Clin Res Cardiol 2018; 107(5): 380-388.
- Smith CM: The therapeutic LDL-C gap. A new oral option. College of Family Medicine, 06/24/2021.
- Drug Information, www.swissmedicinfo.ch, (last accessed Aug. 16, 2021).
- Laufs U, et al: J Am Heart Assoc. 2019 Apr 2; 8(7): e011662. doi: 10.1161/JAHA.118.011662.
- ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT02993406 (last accessed Aug. 16, 2021).
- Stroes ES, et al: Eur Heart J 2015;36(17): 1012-1022.
- Susekov AV, Korol LA, Watts GF: Bempedoic Acid in the Treatment of Patients with Dyslipidemias and Statin Intolerance. Cardiovasc Drugs Ther 2021. DOI: 10.1007/s10557-020- 07139-x; PMID: 33502687.
- Rachamin Yael, et al: Swiss Med Wkly. 2020; 150: w20244.
CARDIOVASC 2021; 20(3): 32-33
HAUSARZT PRAXIS 2021; 16(9): 50-51