The fact that asthma is a very heterogeneous disease is especially true for patients with severe asthma. Additional new drugs are needed mainly for patients whose severe asthma is not well controlled despite standard therapy. More and more biologics are being developed for these patients. Prof. Ian Pavord, MD, University of Oxford, reported on this in Montreux as part of the Joint SSORL/SSAI Meeting 2016. Furthermore, Dr. Thomas Marichal, GIGA-Research Institute Liège, spoke on the topic of aluminum adjuvants in vaccines.
That the new biologics should be used very selectively as adjunctive treatment is illustrated by the results of the first clinical trial conducted with the monoclonal antibody mepolizumab directed against interleukin 5 (IL-5). The cytokine IL-5 is required for the differentiation, maturation, recruitment, activation, and survival of eosinophil granulocytes. Mepolizumab reduces the formation and survival of eosinophils.
Although strong biological effects (e.g., pronounced reduction of sputumeosinophilia) were achieved with mepolizumab, no clinical benefit initially resulted. Based on further studies, it was hypothesized that in patients with severe asthma, eosinophilic inflammation was responsible for asthma exacerbations, and airway dysfunction was responsible for everyday symptoms such as cough and dyspnea. Accordingly, the main benefit of a new drug targeting eosinophilic inflammation is expected to be a reduction in exacerbations.
Mepolizumab clinically effective in properly selected patients
The MENSA study indeed confirmed these expectations [1]. This was a randomized, placebo-controlled, double-blind study involving 576 patients. They suffered from severe asthma, frequently exacerbating despite treatment with high doses of inhaled glucocorticoids, with severe eosinophilic airway inflammation.
Because eosinophil count in induced sputum was found to be replaceable as a biomarker by the much more easily measurable eosinophil count in blood, patients were selected based on blood eosinophilia (blood eosinophil count ≥150 cells per microliter at treatment initiation or ≥300 cells per microliter in the preceding 12 months).
The MENSA trial showed that subcutaneous therapy with mepolizumab (100 mg every four weeks) significantly reduced the exacerbation rate (exacerbations per year) by 53% after 32 weeks compared with placebo and also improved lung function [1].
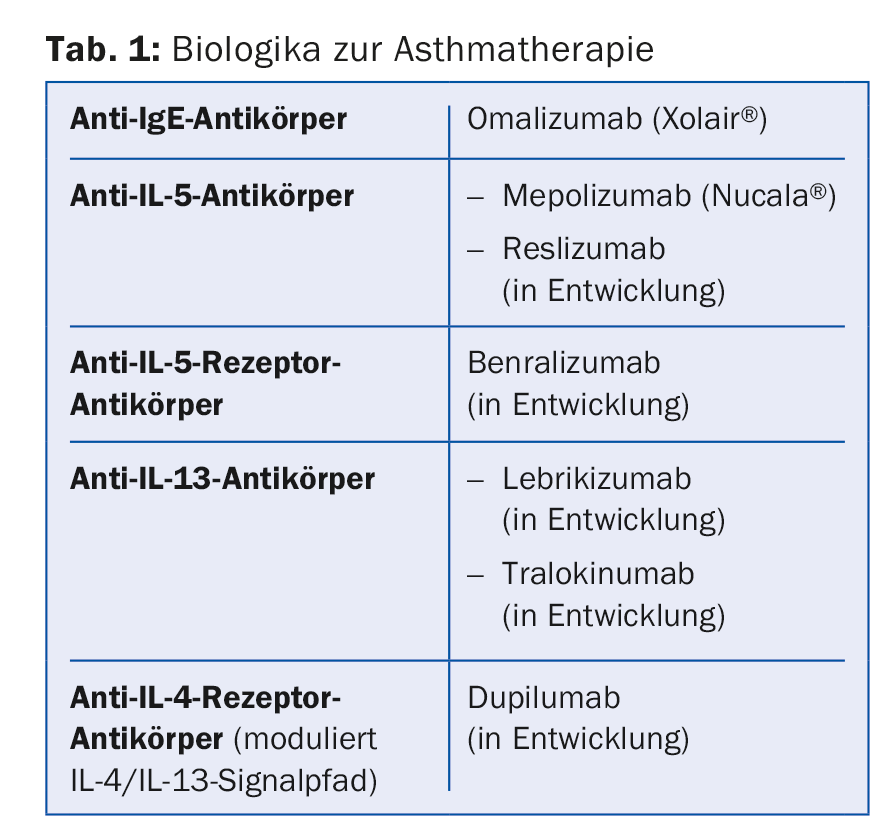
Mepolizumab is the first biologic approved specifically for asthma patients with eosinophilic airway inflammation. Other biologics will follow (Table 1).
The various anti-inflammatory biologics affect biomarkers of inflammation differently, the speaker reported. Anti-IL-5 biologics have a strong effect on blood and sputum eosinophilia but do not affect nitric oxide in the airways (no reduction in fractional exhaled nitric oxide [FeNO]). Dupilumab, on the other hand, reduces FeNO. As easily measurable biomarkers, blood eosinophilia and FeNO provide clear information about the biological events in the airways. IL 5 activity in the airways is responsible for the increase of eosinophils in the blood and because of IL 13 activity in the airways FeNO is increased. Accordingly, it can be speculated that patients with severe blood eosinophilia may be anti-IL-5 candidates and patients with high FeNO may be anti-IL-13 candidates.
Aluminum adjuvants in vaccines
Aluminum compounds (English abbreviation: “alum”) have been used as adjuvants in vaccines for 90 years [2]. Initially, it was assumed that the depot effect of Alum with slowed release of the vaccine antigen from the depot was responsible for the enhanced immune response. However, research results in recent years have shown that Alum is not immunologically inert as originally assumed, but can cause activation of the innate immune system.
When Dr. Thomas Marichal, GIGA-Research Institute Liège, examined alum depots that had rapidly formed at the injection site in animal experiments, he found components of dead cells on the depot surface. Some cytotoxicity of Alum has been known for a long time. Apparently, Alum can kill cells at the injection site, causing them to release their DNA. The innate immune system is activated by DNA released extracellularly. Dr. Marichal was able to show that extracellular endogenous DNA acts as an endogenous immunostimulant and triggers the production of antibodies [3]. Vaccine antigen alone did not induce IgM, IgG1, and IgE antibody formation in mice. However, such antibodies were produced when the antigen was used together with alumadjuvant and also when the antigen was used together with endogenous DNA.
Source: Joint SSORL/SSAI Meeting 2016, April 28-29, 2016, Montreux.
Literature:
- Ortega HG, et al: Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med 2014; 371: 1198-1207.
- Wen Y, et al: Alum: an old dog with new tricks. Emerg Microbes Infect 2016; 5: e25.
- Marichal T, et al: DNA released from dying host cells mediates aluminum adjuvant activity. Nat Med 2011; 17: 996-1002.
HAUSARZT PRAXIS 2016; 11(6): 33-34
DERMATOLOGIE PRAXIS 2016; 26(3): 31-32