The therapy of chronic lymphocytic leukemia (CLL) is currently undergoing rapid change. Increasingly, chemoimmunotherapy, which has been commonly used in the past, is being replaced by more targeted treatments. In addition to new agents such as the BTK inhibitor acalabrutinib, various combination therapies and treatment sequences are also the subject of current studies.
As the most common leukemic disease in Central Europe, CLL particularly affects older people. These could soon benefit from new insights into the optimal treatment strategy. Against the background of demographic change, which suggests a further increase in the number of cases by almost 30% in the next 25 years, advances in therapy are also desirable on an epidemiological level [1].
Focus on signaling pathways
For several years, the B-cell receptor signaling pathway has played a significant role in the treatment of CLL. Malignant growth is highly dependent on its activation and blockade of critical kinases such as BTK (Bruton tyrosine kinase) and PI3K (phosphoinsoditide 3-kinase) can be used therapeutically. The PI3K blocker idelalisib is used primarily in later lines of therapy [1]. BTK inhibitors, on the other hand, have now arrived in first-line therapy as monotherapy or in combination with anti-CD20 antibodies – regardless of mutation status and age (Fig. 1) [1]. While ibrutinib has been approved in Switzerland since 2014, the second-generation BTK inhibitor acalabrutinib has not yet been approved in this country [2]. The European Medicines Agency has given the green light for acalabrutinib in 2020 [3].
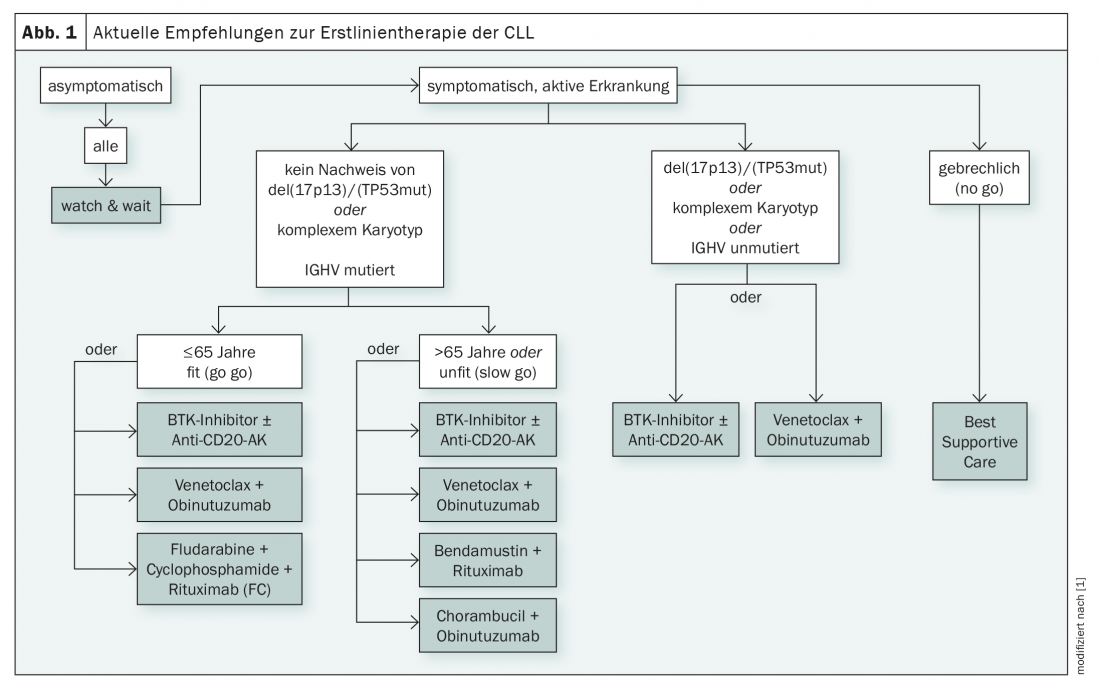
In addition to the B-cell receptor signaling pathway, BCL-2-dependent transduction pathways are also pathogenetically important in CLL. The pro-apoptotic effect of BH3 (Bcl2-homology-domain-3) mimeticsmay counteract this disease-causing mechanism. Venetoclax, a pontent agent, is available here and is used together with the anti-CD20 antibody obinutuzumab in the first line of treatment [1].
With the large number of therapeutic options currently available, choosing the best treatment is not always easy. Complicating matters is a somewhat opaque flood of new data that will likely shape the guidelines over the next several years.
New BTK inhibitors show promise in studies
In particular, interesting data have recently been published on the BTK inhibitor acalabrutinib, which has already led to approval by the European Medicines Agency. While the new agent was tested in previously untreated patients in the ELEVATE TN2 trial, the ASCEND3 trial tested its use in relapsed or refractory CLL [4,5].
In first-line therapy, acalabrutinib as monotherapy as well as in combination with obinutuzumab showed significant prolongation of progression-free survival compared with standard chemotherapy with chlorambucil and obinutuzumab. Combination therapy resulted in a 90% risk reduction for disease progression, and monotherapy produced a corresponding 80% risk reduction. At 24 months, the progression-free survival rate with acalabrutinib-obinutuzumab was 93%, and that with acalabrutinib monotherapy was 87%. In comparison, only 47% of patients receiving standard chemotherapy were still without progression after two years [4].
As in the first line, the new compound also delivered promising results in later treatment lines. In the ASCEND trial, 88% of patients on acalabrutinib therapy remained free of disease progression at one year, compared with 68% in the control arm. Acalabrutinib monotherapy was compared with idelalisib/rituximab or bendamustine/rituximab combination therapy [5]. Consistent with previous findings, both studies showed an acceptable safety profile.
Even if long-term results and overall survival data are still lacking, acalabrutinib is a hot candidate for widespread use in CLL in the near future – also in Switzerland. Currently, more than 70 studies on the new active ingredient are underway. These include studies of applications in other B-cell neoplasms, such as mantle cell or non-Hodgkin’s lymphoma, as well as various drug combinations [6].
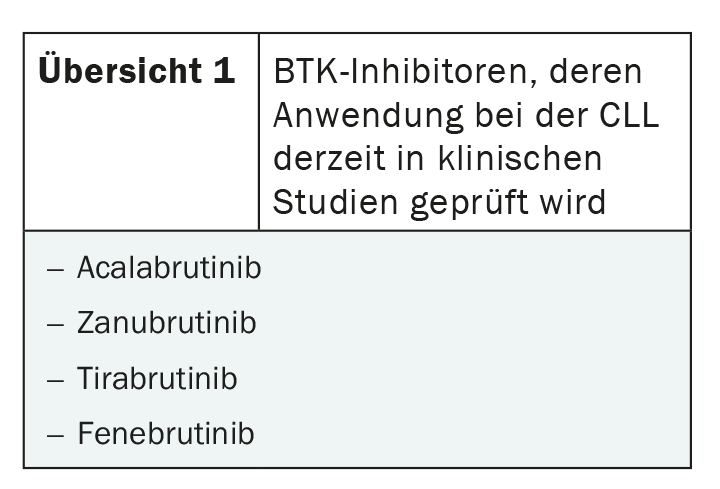
With zanubrutinib, tirabrutinib and fenebrutinib, other new BTK inhibitors exist whose use is currently being tested in CLL (overview 1). A Chinese phase II study concluded that zanubrutinib was well tolerated in later lines of therapy and showed high response rates of 84.6% [7]. Further studies are ongoing, for example for use in patients who have already progressed on BTK inhibitor therapy, but also for first-line use [6,8]. Like zanubrutinib, tirabrutinib has been scrutinized to date, particularly in refractory and relapsed CLL. In combination with idelalisib and with or without obinutuzumab, overall response rates of 60% in the two-drug and greater than 90% in the three-drug combination were observed in the phase II CLLRUmbrella1 study [9]. No results have yet been published on the use of fenebrutinib in CLL, which is currently being investigated in Phase I trials.
What else is in the pipeline?
Not only BTK inhibitors are the subject of current research, but also various other active substances. For example, the first-line use of the interleukin-1 receptor antagonist anakinra is currently being investigated in a phase I trial [10]. This substance, which has so far been used primarily for rheumatoid arthritis, is also not yet approved in Switzerland. Furthermore, studies of the new PI3K inhibitor umbralisib in different disease stages and drug combinations are ongoing [6]. FDA approval for this drug is expected as early as this year [11]. Metformin, the BCL-2 inhibitor navitoclax (ABT-263), various vaccines such as Oncoquest-CLL, IO103, and IO120, the anti-CD20 monoclonal antibody ofatumumab, and several more agents may also soon have a role in treatment [6]. As in other areas of oncology, immunotherapeutic approaches to the treatment of CLL are being investigated, including the bispecific antibody epcoritamab [12].
With the overwhelming amount of potential and already approved new agents for the treatment of CLL, the question of optimal therapeutic combinations and sequences, as well as biomarkers to select the best possible patient population, is increasingly raised. Diligent research is currently being conducted in this regard, and initial findings can be expected in the near future.
Stem cell transplantation soon obsolete?
The only curative approach in CLL remains allogeneic stem cell transplantation, yet this is increasingly taking a back seat with the availability of highly effective molecular therapies. Today, it is only an option in situations that are prognostically unfavorable even with the newly available treatment options. The development of innovative agents and the investigation of different therapeutic regimens may further niche allogeneic stem cell transplantation in CLL in the near future. Let’s hope so.
Literature:
- Wendtner C-M, et al: Chronic lymphocytic leukemia (CLL) – Onkopedia. September 2020. www.onkopedia.com/de/onkopedia/guidelines/chronische-lymphatische-leukaemie-cll/@@guideline/html/index.html (last accessed Jan. 24, 2021).
- swissmedic swiss Agency for Therapeutic Products: Arzneimittelinformation. www.swissmedicinfo.ch/ (last accessed on 24.01.2021)
- EMA: Calquence. www.ema.europa.eu/en/medicines/human/EPAR/calquence (last accessed on 24.01.2021)
- Sharman JP, et al: Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): a randomised, controlled, phase 3 trial. Lancet. 2020; 395(10232): 1278-91.
- Ghia P, et al: ASCEND: Phase III, Randomized Trial of Acalabrutinib Versus Idelalisib Plus Rituximab or Bendamustine Plus Rituximab in Relapsed or Refractory Chronic Lymphocytic Leukemia. J Clin Oncol. 2020; 38(25): 2849-61.
- ClinicalTrials.gov. www.clinicaltrials.gov (last accessed on 24.01.2021)
- Xu W, et al: Treatment of relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma with the BTK inhibitor zanubrutinib: phase 2, single-arm, multicenter study. Journal of hematology & oncology. 2020; 13(1): 48.
- Tam CS, et al: Zanubrutinib monotherapy for patients with treatment naïve chronic lymphocytic leukemia and 17p deletion. Haematologica. 2020; Epub online ahead of print.
- Safety and Efficacy of the Combination of Tirabrutinib and Idelalisib With and Without Obinutuzumab in Adults With Relapsed or Refractory Chronic Lymphocytic Leukemia (CLL) – Study Results – ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/results/NCT02968563 (last accessed on 24.01.2021)
- Anakinra in Previously Untreated Chronic Lymphocytic Leukemia Patients – Full Text View – ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04691765 (last accessed on 24.01.2021)
- Rai-Roche S.: TG Therapeutics’ umbralisib, ublituximab likely to face market hurdles but FDA approval highly expected. Pharmaceutical Technology. 2020. www.pharmaceutical-technology.com/comment/tg-therapeutics-umbralisib-ublituximab-likely-to-face-market-hurdles-but-fda-approval-highly-expected/ www.pharmaceutical-technology.com/comment/tg-therapeutics-umbralisib-ublituximab-likely-to-face-market-hurdles-but-fda-approval-highly-expected/ (last accessed Jan. 24, 2021).
- Safety and Efficacy Study of Epcoritamab in Subjects With Relapsed/Refractory Chronic Lymphocytic Leukemia – Full Text View – ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04623541 (last accessed on 24.01.2021)
InFo ONCOLOGY & HEMATOLOGY 2021; 9(1): 24-25.